Soil pH
What is pH?
pH Basics
For anyone who might be having painful flashbacks of high school chemistry, there’s good news. pH is a really simple concept: It’s just the quantity of hydrogen atoms (H+) in a given place. That’s it. Very acidic conditions mean lots of H+, while very basic conditions mean very few H+. The pH scale goes from 0 (extremely acidic) to 14 (extremely alkaline), meaning 7 is neutral. A soil of 5.5 pH is considered acidic and has more H+ than one at a pH of 8, which is “basic” or “alkaline”.
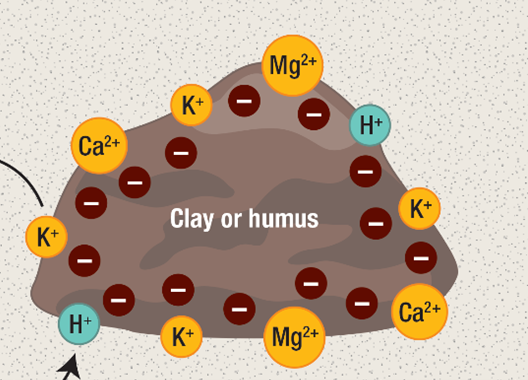
In the soil, these H+ can be found in one of two places: floating in the water or clinging to soil solids like organic matter or clay, but less so with sand and silt. Soil solids have negative charges on their exterior, so the positive charge of the H+ sticks to soil exactly like the positive and negative poles of magnets stick together. The soil has plenty of other positively charged nutrients that do the same, including calcium (Ca2+), magnesium (Mg2+) and potassium (K+). Anything with a positive charge is called a “cation” in science-speak. (Just remember: Cat-ions have paws-itive charge) You may have heard of a soil’s “Cation Exchange Capacity” (CEC). This just refers to the total number of negative sites in the soil that cations can magnetically stick to. In many ways, cations compete with each other for these negatively-charged sites and can bump each other off, which is essentially how lime raises pH. More on that later.
pH (a.k.a. the amount of H+ hanging around) is important to quantify because the compounds that make up living things begin to dissolve and break apart outside of a certain pH range. This is why the human body regulates blood pH to a miraculously tight range: 7.35-7.45. It’s also why our stomachs employ an acid with a pH of 2 to dissolve and break down the food we eat.
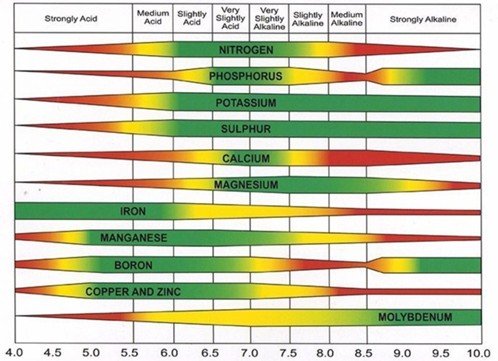
Without diving too deep into the controversy, traditional agronomic thinking is that nutrients become more or less available depending on pH. It’s taught that essential nutrients like iron (Fe), manganese (Mn), Boron (B) and aluminum (Al) are more available in acidic conditions, meaning they can build up to toxic levels in plants growing in low pH soils. Essential nutrients like molybdenum (Mo) and magnesium (Mg) are believed to be more available in basic conditions, meaning toxic levels can build up in plants growing in high pH soils. Innovative technology and experimentation are challenging this simplistic model of nutrient availability, meaning nutrient availability charts, like the one presented below, are likely overly simplistic.1
Natural Soil pH Levels
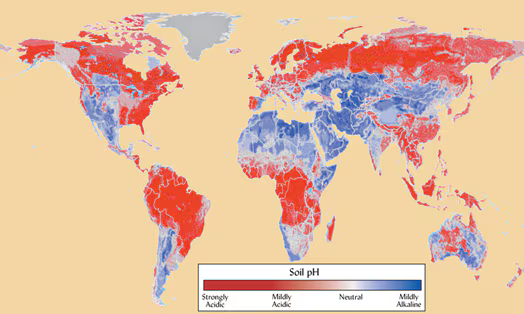
The truth is that soils around the world vary in their natural pH, largely related to annual rainfall. In higher rainfall areas, such as in the eastern US and land near the equator, soils slowly, but naturally, acidify. This occurs for a couple reasons. First, microbes excrete acids after they consume food. Those of you who have had cavities know this firsthand. Acids produced by bacteria in the mouth slowly decay tooth enamel. Sugary foods fuel microbial activity and result in more acid production, which is why candy does what it does to teeth. In the soil, moisture is often the limiting factor to microbial activity, so rain leads to a flurry of microbial feeding, which leads to acid production. In addition, microbes breathe out carbon dioxide (CO2) just like all living things, so more microbial activity means more is released.
This CO2 combines with water (H2O) to become a weak acid called carbonic acid (H2CO3), which can build up in the soil. All of these acids are not necessarily a bad thing. In fact, they are the reason why many nutrients are continually dissolved from rocks in the soil and made plant-available year after year. In addition, most agronomists agree that soils are more productive in the 6.3-6.8 range, meaning a slightly acidic environment. But the point here is soil moisture = more microbial activity = more acids.
Second, rain is naturally slightly acidic and adds H+ to the soil. Acid rain falling downwind of industrial processes acidifies soils much quicker, as was observed during the 19th and 20th centuries. Additional H+ from rain bumps positively charged nutrients like calcium (Ca2+), magnesium (Mg2+) and potassium (K+) off soil particles and leaves them at risk of leaching down through the soil and into the watershed. Losing these positively charged nutrients gives H+ more of a free reign and a chance to build up over time.
In contrast, soils in environments with less rainfall, such as in the western US, are at risk of slowly becoming alkaline. This is because less H+ is added to the soil from the rain. Low rainfall also means positively charged nutrients that compete with H+ do not leach out of the soil as quickly as they are dissolved from rocks in the soil by microbes. Therefore, the amount of calcium (Ca2+), magnesium (Mg2+), potassium (K+) and even sodium (Na+) increases over time and bumps out H+, resulting in a rise in pH.
Manmade Soil Acidification
The processes listed above naturally take a long time to play out and find an equilibrium. On the other hand, conventional management of cropland and pastureland has acidified many millions of acres unnaturally quickly in the past century or so, and this is the real issue at hand.
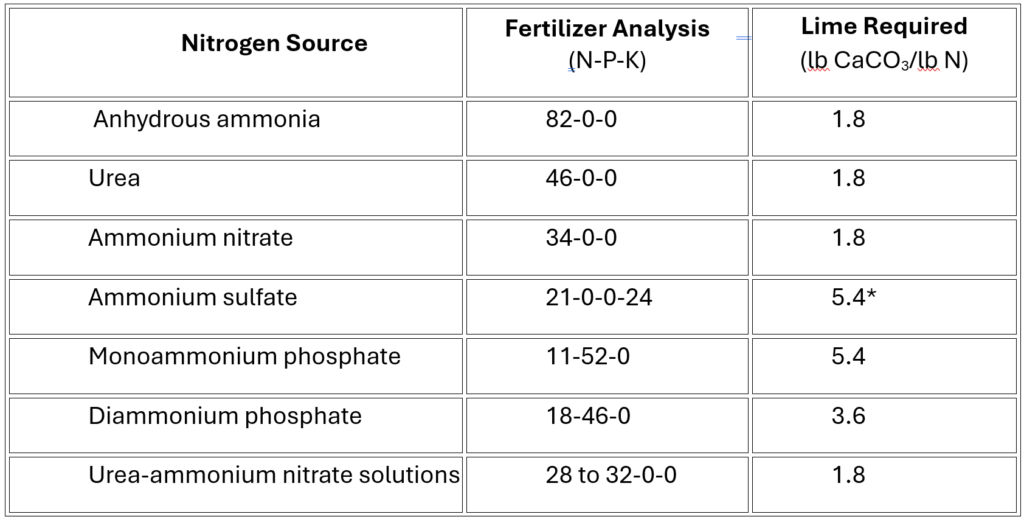
One cause of self-inflicted soil acidification is the excess use of nitrogen fertilizers like ammonium sulfate and urea because two H+ are released each time an ammonium (NH4+) ion is converted to nitrate (NO3–). This process happens in the soil naturally all the time, but the enormous dump of nitrogen fertilizer throws the process into hyperdrive, producing a large amount of H+. In addition, ammonium sulfate is broken down into sulfuric acid (H2SO4), a strong acid. Lastly, ever increasing crop yields over the decades has meant more calcium (Ca2+), magnesium (Mg2+) and potassium (K+) leaves the field at harvest, which has the same effect as them leaching in high rainfall areas because H+ is allowed to reign, and the balance of positively charged nutrients is thrown out of whack.
Various forms of nitrogen fertilizers affect soil pH differently, as can be seen in the chart below from North Dakota State University which shows how much lime is required to neutralize various fertilizers.2
Soil acidification also occurs in fields with high quantities of legumes like soybean and clover because enormous flushes of ammonium (NH4+) nitrogen are added to the soil when they decompose. This is yet another reason to prioritize diversity of species in cash crop rotations, cover crop mixes and pasture composition.
Another cause of soil pH problems is the slow loss of organic matter. Interestingly, organic matter does have a slightly acidifying effect as microbes decompose it and produce organic acids. However, as mentioned before, organic matter also provides crucial buffering effects of soil pH thanks to its numerous negatively charged sites that magnetically bind to H+ and other positively charged nutrients. Huge flushes of nitrogen or H+ don’t have as big of an effect on pH with high organic matter because they join a huge reservoir of positively charged nutrients already present. This is like putting $100 in a bank account with $100 versus putting $100 into a bank account with $100,000. The change in the first account is enormous but is only a small addition in the second. Such is the case with organic matter and its ability to moderate pH.
Soil organic matter also feeds and houses the trillions of microbes living in the soil that build soil structure. Proper soil structure contains macroaggregated soil particles and sufficient pore space for air and water exchange. These characteristics create a plethora of conditions in the soil, including areas that are dry, wet, anaerobic, aerobic, large, tiny and everything in between. A diversity of environments leads to an abundance and diversity of living creatures that thrive in each unique niche. Research in the area of microbial mediation of pH is in its infancy, but organic matter is known to improve problems with soil acidity3 and I suspect that a large portion of this occurs as a result of nutrient balancing and cycling by the trillions of living organisms working together in all of the different niches.
Manmade Soil Alkalinization
As mentioned previously, arid environments tend to produce alkaline soils over time because the quantity of cations like calcium (Ca2+), magnesium (Mg2+), potassium (K+) and even sodium (Na+) increases over time and bumps out H+ on negatively charged sites in the soil. Unfortunately, historical and current management by farmers and ranchers in these environments has sped up these nutrient buildups. The most common cause for these buildups is the excessive use of irrigation. Essentially, irrigation water from below contains a high concentration of these nutrients from the surrounding bedrock. This irrigation water lands on the surface and either gets taken up by plants or evaporates, leaving the nutrients behind in the form of white, salty material. This is the same reason why white specs of salt remain on our body after our sweat evaporates away. Similarly, another risk factor for the dangerous buildup of these nutrients and salts is poor drainage. High levels of these salts inhibit microbial and plant life over time.

Another management practice that has driven up soil pH and salt levels in arid regions is the replacement of deep-rooted perennial species with annual crop varieties. The reason is annual crops, along with fallow periods, decrease the amount of groundwater that is released through plant evapotranspiration. Over time, this causes the water table to rise, increasing the flow of nutrient-rich groundwater to low spots. This water will increasingly move toward the surface and results in high pH/salt buildup situations. As it stands, nearly 40 million acres of cropland and pasture in the western United States is negatively affected by buildup of these nutrients, and that number is growing annually.4
With a basic (no pun intended) understanding of pH under our belts, the subsequent section will discuss how lime works to works to raise soil pH in acidic soil conditions. In addition, it will cover how soil structure, nutrient balance and overall function are affected by this nearly universal amendment.
Acidity & Liming
The first section of this article outlined the basics of soil pH and how management practices can push soil to become more acidic or more alkaline. This section will investigate lime, the universally recommended amendment given to farmers and ranchers working with acidic soil conditions. Understanding the basics of lime will empower farmers and ranchers to make better purchasing decisions and avoid relying solely on the recommendations of someone else. Given these trying economic times, producers need to think long and hard about which products are making money and which are just expensive band-aids.
What is Lime?
Many farmers and ranchers are familiar with agricultural lime, having literally purchased and applied tons of it to their ground to moderate soil pH, as well as other purposes like improving soil structure and balancing fertility levels. In fact, spreading lime is a very old practice that farmers have undertaken for centuries.5 But what exactly is lime and how does it work?
Agricultural lime is essentially crushed limestone or chalk. Limestone is a category of rock composed mainly of calcium carbonate (CO32-) that forms in various locations, including deep-sea beds, shallow water and caves. Limestone can be made of many different minerals in combination with CO32- and can be found in many different colors and textures. Magnesium is one such element that is commonly found in limestone alongside calcium. In my home state of Indiana, limestone quarries dot the southern half of the state and provide excellent building material, which is why it’s been used for 35 of 50 state capital buildings, the Empire State building and the Pentagon. In the United Kingdom where I currently live, many fields and pastures have a few inches of topsoil before hitting a deep layer of a specific type of limestone: chalk. The Cliffs of Dover in the Southeast of England provide a good visual to show just how thick these chalk layers can be compared to the tiny layer of soil on top that sustains life.

Chalk is a soft limestone that accumulates in shallow marine environments from the skeletal remains of sea creatures. Just like calcium is an important constituent of our bones, sea creatures like plankton and algae use calcium to make hardened shells. To do this, they absorb free-floating calcium (Ca) and combine it with a compound called carbonate (CO3), a byproduct when carbon dioxide and water combine. The end result is “calcium carbonate” (CaCO3). The image below shows a microscopic view of a certain type of plankton and its intricate shell, which forms a significant part of chalk:

Once creatures like plankton die, they and their shells sink to the bottom and layer on top of each other, which is why you can see very distinct horizontal layers, or “stratification” in the Cliffs of Dover image. This is also why you can see the remnants of little sea creatures when looking at many types of limestone and chalk under a microscope, such as in the thin slice of limestone below:
You’ve probably heard the statistic that there is more carbon in the soil than in all the plant life and atmosphere combined. While this is impressive, Earth’s crust is estimated to hold around 75 million (75,000,000!) Pg of carbon in the form of carbonate rock, making it by far the biggest pool of carbon on the planet. By comparison, soil holds a miniscule 3,000-4,000 Pg, vegetation holds 640 Pg and the atmosphere holds 790 Pg. (1 Pg is 1,000,000,000,000,000 g) This means calcium carbonate shells sinking to the ocean floor is a very important part of Earth’s carbon cycle.
Carbon in the rocky reservoir reaches the surface once again in the form of carbon dioxide from volcanic eruptions or as bodies of water dry up and the floor is exposed to the air. An extremely long time passes in either case. That is, unless we humans hit the fast forward button and extract it from the ground, such as what happens when limestone or chalk is mined.Once mined and ground down, there are two basic types of agricultural lime: dolomitic lime (high calcium and high magnesium) and calcitic lime (high calcium and low magnesium). The difference simply comes down to how much magnesium was in the original limestone or chalk. Just as there are veins of gold and other elements in specific places around the planet, magnesium may or may not be rich in the limestone where it was mined.
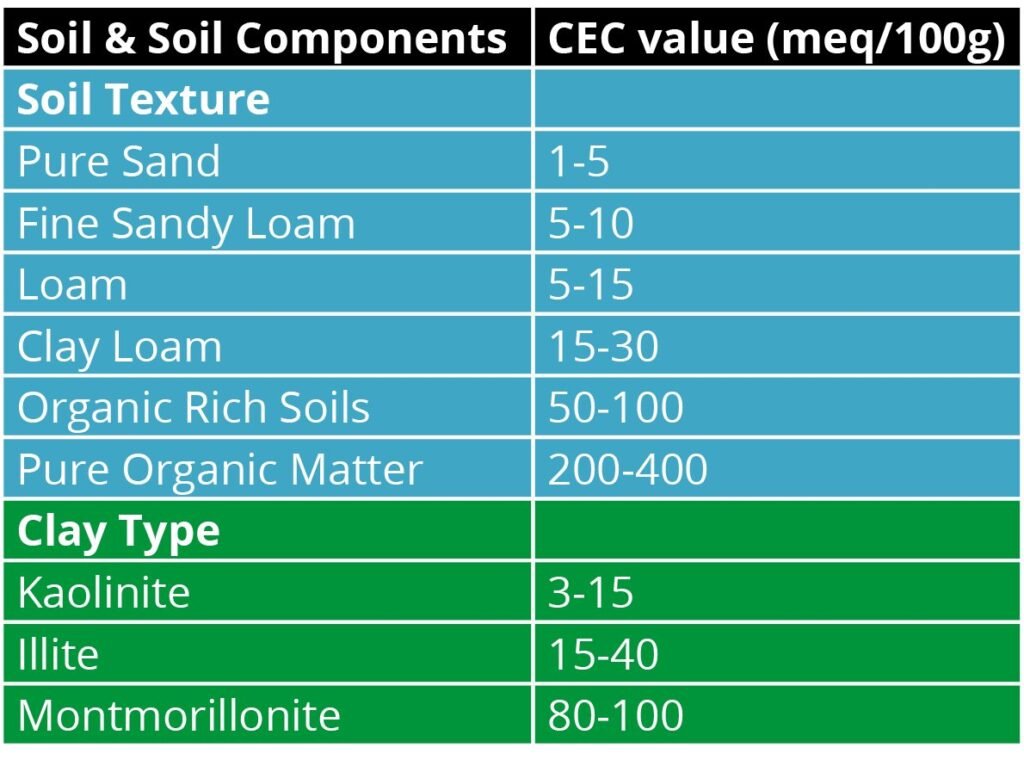
Lime is used in both agricultural and industrial processes, including wastewater treatment and sugar beet processing. Farmers and ranchers can often purchase and apply this “spent lime.” Some get it for free. Wood ash is another amendment with the capability of raising pH. In any case, determining the necessity, type and amount of lime is dictated by soil pH, soil fertility levels and cation exchange capacity (CEC).
How Does Lime Change pH?
To briefly review, pH is simply the quantity of hydrogen atoms (H+) hanging around. Very acidic conditions mean lots of H+, while very basic conditions mean very few H+. Lime is applied to neutralize and/or dislodge these H+ from soil particles where they can be leached out of the soil. Lime also dislodges aluminum (Al3+) nutrients that indirectly acidify soil by releasing any H+ it is attached to.
Before lime can work, it needs to fully dissolve in soil water, so the finer the size of the lime, the quicker it will get to work. This is analogous to a large ice cube melting slowly in water versus the same amount of crushed ice cubes melting much quicker. Very fine lime or liquid lime applications can begin working in a little as a few weeks, while coarser lime can take a year to result in an appreciable change in pH. The effects of a typical application last around 3-5 years depending on the fineness and amount.
After lime fully dissolves, 2 H+ are wrangled by oxygen (O) and become part of a harmless water molecule (H2O). Voila! Fewer lone H+. The full reaction results in three products in total, which are water, carbon dioxide and calcium (Ca2+) or Mg (Mg2+). These calcium (Ca2+) and Mg (Mg2+) can bump additional H+ or aluminum (Al3+) off negatively charged soil particles and leave them vulnerable to becoming neutralized or leached deeper in the soil. As a general rule of thumb, 1 ton per acre of lime will raise pH by 0.3 units on a medium textured mineral soil after 3-5 years. Soils with more clay and/or organic matter require more lime to have the same effect thanks to their higher CEC.
Observed Benefits of Lime
Many studies have been undertaken which show the ability of lime to raise soil pH and, consequently, plant productivity. One reason is that a complete range of essential plant nutrients are more available in a pH zone of 6.3–6.8, roughly speaking. In addition, most plant roots are significantly stunted in acidic soils due to excess aluminum (Al3+) availability, which leads to toxicity. Finally, the addition of readily plant-available calcium and magnesium can provide a much-needed shot in the arm to plants that lack access to these crucial nutrients. Magnesium is, after all, the central nutrient in chlorophyll!
Calcium and magnesium are also important nutrients in the soil because they affect the amount of pore space. Calcium is able to clump clay particles together, which creates pore space for air and water, like marbles in a jar. On the other hand, magnesium disperses clay particles and does not allow them to clump tightly. This causes the soil to behave like flour that has water poured on it. Although the particles are fine and spread out, there are not enough large pore spaces between them to let water infiltrate easily. Proper soil function requires zones of soil aggregation surrounded by larger pore spaces for air, water and organisms to move about freely.
This is why many soil scientists and agronomists believe there is an ideal ratio of calcium and magnesium (along with other positively charged nutrients like potassium (K+), sodium (Na+) and even H+) that is necessary for proper soil structure and function. Others, like Dr. Ray Weil of the University of Maryland, disagree with the idea that there are ratios to chase, saying, “This belief is based on a few out-of-date research studies from the mid-20th century and can lead to the wasteful use of soil amendments in an effort to achieve the so-called “ideal” calcium to magnesium ratio.”6
Is Lime Worth It?
Farmers and ranchers are constantly bombarded with conflicting information like the above from experts on every possible topic, so who do we listen to? Unfortunately, it’s no coincidence that most of the results for my Google search on “Benefits of liming soil” took me to web pages of companies that have a financial incentive to sell more lime. That’s just the world we live in and, hey, more power to them. This isn’t always the case, but many times it is. Therefore, I contend, as do my colleagues at Understanding Ag, that you are best served in the long-run by listening to nature.
We recommend doing trials on your own farm or ranch to understand the effect of any product, and lime is no different. This is the gold standard for attaining ROI on your operation. A simple example would be to split a ten-acre plot into thirds: one third receives the recommended rate of lime, one third receives a half rate and one third receives none as a “control” to compare against land that gets lime. Then, observe what happens in subsequent growing seasons. How does pH differ between the test plots? Was there a significant yield drag? Does plant species composition differ? Which plot had the highest net returns? Conduct trials like this on as much land as possible that will allow you to sleep comfortably at night. You’ll be glad you did. The knowledge gained could save hundreds of thousands of dollars over your farming or ranching career.
Arguably more important than running trials is to think about practices that could be acidifying the soil in the first place and brainstorm ways to eliminate or reduce them in your operation. Yes, it’s a natural process in high rainfall areas, but management practices like synthetic nitrogen fertilization, chronic tillage and overgrazing could very well be supercharging acidification, leading to the need for lime every 3-5 years. The fact is that many Understanding Ag consultants have personally reduced or eliminated lime applications in their own operations after making management changes. Many of our clients around the world have done the same. At the end of the day, agricultural lime is just another tool in the toolbox. It can be seen as a short-term amendment that helps a soil reach a healthy equilibrium or it can be seen as a never-ending, no-questions-asked expense that temporarily raises pH until more is needed. How you view it is up to you.
The following section will discuss alkaline soil management, the opposite issue that many producers in arid regions face. Specifically, it will unpack how various amendments and practices lower pH and whether they are worth the investment.
Alkalinity
Soil acidification is a well-publicized topic of concern in scientific and governmental circles, but alkaline soil conditions (soils above 7 pH) are every bit as challenging to farmers and ranchers who deal with them.
Third Rock from the Sun
Excuse the pun, but geology rocks. Farmers and ranchers have a special relationship with geology whether they know it or not. The sand, silt and clay particle that make up soil are all tiny bits of weathered and degraded rock. The vast majority of nutrients their plants and animals need come from rocks, with the exception of nitrogen and carbon (from the air), as well as hydrogen and oxygen (from water). Everything else like phosphorus (P), potassium (K), boron (B) and all the rest were once wriggled free from the tight grips of rocks. This wriggling, or “weathering”, of nutrients is a never-ending process that resupplies soil with usable nutrients. Both non-living forces (like water) and living forces (like microbes) can weather nutrients out of rock, which increases the total pool size of nutrients available for living organisms to use. Old headstones are testament to this process.

There is an important opposing force that causes nutrients to exit the soil, which is called “leaching.” Water is the main culprit in this process because it has an uncanny ability to magnetically grab nutrients and take them wherever it goes, either off the surface or down through the soil into the water table where plants can no longer use them. Natural ecosystems tend to strike a balance between weathering and leaching when precipitation and plant water use are similar. However, we already know that some environments have high precipitation, which leaches away more nutrients than are weathered, causing H+ to build and soil to acidify.
In dry environments, more nutrients are weathered from rock than are leached away, causing the soil to become more alkaline. This is because calcium (Ca2+), magnesium (Mg2+), potassium (K+) and sodium (Na+) compete with H+ and aluminum (Al3+) by bumping them off negatively charged soil sites. H+ and aluminum (Al3+) are then prone to leach away or become neutralized. In addition, the positively charged nutrients listed above can react with soil water and produce the counterpart to H+, which is OH–. OH– drives pH up, making conditions more alkaline, sometimes to a level that hinders plant productivity. This is no small issue, as around 45% of Earth’s land is considered arid or semiarid, so rising soil pH is a concern for producers across the globe.
Salt of the Earth
What if a producer has the capability to apply irrigation water? Will this simulate precipitation and fix the problem? The short answer is no. Irrigation exacerbates the alkaline problem in most cases because irrigation water is chock full of these weathered nutrients, particularly when it is sourced from river water, a common practice in the American West. Applied irrigation water is subsequently used by plants or evaporated away, leaving many of the nutrients behind. These positively charged nutrients can cling to negative sites in the soil, but they may also cling to negatively charged nutrients to become a salt. Salt, by definition, is the combination of a positively charged and negatively charged nutrient. Think about the salt we use to flavor our food: sodium (Na+) and chloride (Cl–) make sodium chloride(NaCl) or table salt. As a result, many irrigated landscapes are left with white, powdery salts in and on the soil.

Plants and microbes are severely hindered when excess salt levels build up, which is why ancient civilizations would “salt” the agricultural fields of their rivals as a wartime tactic.7 Problems also occur downstream, as water leaving irrigated fields is filled with unnaturally high levels of salt. For example, the Colorado River contains a very high concentration of salts by the time it reaches the U.S.-Mexico border, thanks to upstream irrigation systems and industrial use. As a result, a very large and expensive desalinization plant had to be built at the border to ensure clean water was entering Mexico.8
As mentioned in the first section of this article, rising water tables are another cause of rising soil pH/salt accumulation. In this case, nutrient-rich groundwater reaches the soil surface as the soil wicks it upward. This effect is first observed in low spots of the land. Loss of deep-rooted perennial species is a major cause of water table rise in these arid environments. Annual plants are simply not able to draw up and evapotranspire groundwater as efficiently as perennial species, so the water table slowly accumulates and climbs. Introducing fallow periods into a cropping rotation also speeds up water table rise in these environments.
Salty landscapes also form naturally when bodies of water slowly dry up and leave their salts behind, such as at the Bonneville salt flats in Utah. In total, salt-affected soils cover approximately 7% of the total land on Earth, 23% of cultivated agricultural land and nearly 50% of irrigated land.9
Lowering pH
Removing excess H+ in an acidic soil is fairly straightforward with the availability and action of lime. Removing excess salts in an alkaline soil is a little more challenging. The good news is that salts dissolve in water, ie. they are “water soluble”. This means they will readily hitch a ride in the water and go wherever it goes. Here lies a root cause of high pH. Many agricultural soils are compacted and have poor drainage, so water and the excess salt do not flow through properly. This is why tile drainage is pushed by conventional agronomists in these situations. Perforated tile speeds up the movement of water out of the soil, taking the salts with it.

Oftentimes, nutrients like magnesium (Mg2+) and sodium (Na+) are not in the form of leachable salts and need to be transformed into one so water can carry them away. This is why another common piece of advice is to apply some form of sulfur amendment. Common choices are gypsum (calcium sulfate) and elemental sulfur. Gypsum is used because it combines excess magnesium (Mg2+) with sulfate, making a salt called magnesium sulfate (a.k.a. epsom salt). In a similar way, excess sodium (Na+) becomes a salt called sodium sulfate. Once again, this strategy works best in soils that receive adequate moisture and exhibit good drainage to flush the newly formed salts away. Finally, elemental sulfur is recommended because it turns into sulfuric acid, a very strong acid with a pH around 2-3. This means sulfuric acid readily donates H+ to the soil, which lowers pH.
Organic Matter
One often overlooked strategy to bring down high soil pH is to build organic matter levels. As you might recall, soil organic matter behaves as a powerful pH buffer due to the high number of sites that can bind with nutrients like calcium (Ca2+), magnesium (Mg2+), H+ and all the others. This characteristic positively influences soil nutrient balance over time. In addition, organic matter decomposition by microbes can help lower pH thanks to the weak acids they directly exude, as well as the carbonic acid created from the carbon dioxide they breathe out. (See Part 2 for more on this process.) Scientists don’t have a full explanation of how these processes play out, but they do know that soil nutrients and pH move toward a healthy range when organic matter levels and the richness and diversity of soil life increase. When allowed, nature is self-healing, self-organizing and self-regulating.
Higher organic matter levels in the soil also fight against high pH because organic matter, and the soil organisms that it feeds and houses, actively improve drainage and a soil’s water holding capacity. In other words, a good balance is struck between water infiltration, water holding capacity and water percolation down the soil profile. Drainage is improved through the action of microbes that create proper soil aggregation, as well as increased earthworm and insect tunnels that water containing excess salts can use to percolate downward. Water holding capacity is increased because organic matter behaves like a sponge, magnetically biding to polar water molecules in a way that plants can access. This reduces the need for irrigation water when conditions start to turn dry.
An important point to always remember about organic matter is that its accumulation and decomposition happen at the same time, sort of like a person’s monthly income brings in money and monthly expenses cost money. This means building organic matter levels requires putting in a little more organic matter than is decomposed every year.
Farming and ranching on soil with a high pH can be challenging, particularly when excess salts have accumulated. Installing drainage tile and applying gypsum are often recommended to rectify the situation for their ability to improve drainage and flush away salts. It’s easy to see where advocates for these practices are coming from and, in fact, many farmers and ranchers have lowered soil pH by implementing such practices. But these amendments and practices are tools, not magic bullets. Soil pH will revert back to undesirable levels if they aren’t accompanied with systematic changes as well. The final section in this article will look at soil pH management from a regenerative perspective, highlighting farmers and ranchers who have significantly reduced or eliminated the need for inputs that temporarily raise or lower pH.
Regenerative pH Success Stories
Introduction
Someone wise once said, “We’re not managing problems in agriculture anymore. We’re managing solutions.” It’s true that many products and practices widely adopted over the past 100 years intended to solve a problem have created new problems, sometimes more challenging than the ones they were designed to conquer. In the field of medicine, these are called side effects. In agriculture, we call them unintended consequences, and throwing soil pH out of whack with well-intentioned products and practices is one of the more common unintended consequences Understanding Ag consultants observe across North America and beyond.
The final installment in this “Understanding pH” series will highlight various examples of farmers and ranchers who have changed their management system in such a way that they are reaping the reward of positive unintended consequences. (Who knew those existed?) These farmers and ranchers are saving serious time and money because, in their own words, “pH is something I just don’t worry about anymore.”
Cropland to Pastureland
As discussed in part 2 of this series, many conventional cropping practices acidify the soil unnaturally quickly. The reasons vary, but common culprits include excessive N fertilizer use and the loss of organic matter. What better way to eliminate the need for N fertilizer and build organic matter levels than to temporarily or permanently introduce pastureland and grazing animals back to cropland. This is exactly what Jay Fuhrer and the team at the Menoken Farm did starting in 2013.
The Menoken Farm is located near Bismark, North Dakota in the Great Plains region of the U.S. This once vast grassland ecosystem has been a hotbed for cropland conversion in the 21st century. In fact, from 2008-2016, croplands expanded at a rate of over one million acres per year, with the eastern half of the Dakotas leading the charge. Unfortunately, 69.5% of new cropland areas produced yields below the national average, with a mean yield deficit of 6.5%.1 With this in mind, Fuhrer and the team at Menoken decided to transition conventionally managed cropland back into grazing land. Cropland was seeded back to grass in 2014, and cattle were grazed during the 2015, 2016 and 2017 seasons. No amendments were applied during that time, just sound grazing principles.
The results of the trial were impressive. As you can see below, pH in the eastern half of the trial rose from 5.8 to 6.8, while organic matter rose by 0.4%. The western half of the field’s pH rose from 5.8 to 6.2, while organic matter nearly doubled from 2.0% to 3.7%. Again, these results were achieved through managed grazing of cattle without the purchase and application of any fertilizer or pH amending products.
Image 1: pH and soil organic matter (SOM) changes on Menoken farm after five years of conversion from cropland to grassland with adaptively managed grazing.
Many farmers do not have the resources or desire to permanently revert cropland back into grazing land. For them, a temporary break from cropping might be a good option. In the UK, it is common practice to introduce “leys” into the cropping rotation. This simply means turning cropland into temporary pastureland for roughly 2-5 years and back to cropping. British farmers have observed positive results that resemble those observed at the Menoken Farm, including pH balancing, organic matter accumulation, weed suppression and yield bumps in subsequent cash crops. Many farmers also note an increase in earthworm numbers, which is important because earthworm castings and their slimy mucus that coats the soil typically has a pH of 7 and is chock full of H+-competing cations like calcium (Ca2+) and potassium (K+).
Cover Crops
If putting ground into multiple years of pastureland is not a viable option, planting cover crops can be a great short-term practice for increasing overall soil health and improving soil pH naturally. Take David Brandt, often described as a godfather of the soil health movement. In 1971, the soils on his family farm in Ohio contained 0.75% organic matter and a pH as low as 4.2 in some spots. He applied two tons of lime per acre that year, but did not apply any more lime after that. By focusing heavily on implementing cover crops, no-till management and diversity of plant species, Brandt and his family completely transformed their central Ohio soils. In 2022, the farm averaged 8.7% organic matter and a pH of 6.9.2 Again, no lime was applied since that initial 1971 application. In contrast, neighboring farm ground he purchased in 2016 contained only 1% organic matter, showing that management makes all the difference.
Image 2: Rolling down and planting into cover crops at Brandt Farm
On the flip side, Understanding Ag consultant Kent Solberg helped a North Dakota producer turn around high pH cropland soil. The farmer was trying to produce crops on a 30-acre alkaline area in a 150 acre field that he hadn’t grown a cash crop on in over 20 years, only kochia. Kent custom designed a full season complex cover crop mix and strip grazed it with stocker cattle in the late summer-early fall timeframe. They carefully selected cover crop species that were tolerant to high pH soils. The farmer planted the field to oats the following year. To his delight, the oats actually grew in the alkaline area. The farmer estimated a 160+ bu/acre potential yield for the field before the oats were unfortunately flattened by a hail storm. Regardless of the hail loss, the farmer was so excited that those 30 acres actually produced a crop.
Better Pastureland Management
While pastureland is typically easier to manage for soil pH than cropland, it is still susceptible to soil imbalances. Poor grazing management can easily throw off soil pH and hinder overall functioning of the soil ecosystem. Like cropland, excessive fertilization and disturbance of the soil can acidify the soil in a hurry. In this case, tillage is less of the disturbance in question, although some pastureland is regularly disturbed mechanically. Rather, overgrazing of the land is the main culprit. Pastureland that is overgrazed throws the system out of balance because root mass deteriorates on a large scale, organic matter is removed faster than it is replenished, the soil compacts and water cycles inefficiently, among other reasons.
Adaptively grazed pasture remediates pH because it allows living plants the opportunity to feed the soil for an extended period of time. Plant tissue, including roots and shoots, as well as the compounds they produce, are the origin of all organic matter in the soil. Organic matter is what feeds and houses the trillions of soil inhabitants that work tirelessly to balance the soil ecosystem. A steady pH relies upon a balanced soil ecosystem.
In researching stories for this article, many examples abound of soil health improvements observed when a system is transitioned to a more adaptive, multi-paddock grazing system. The first success story comes from Otter Creek, New York, where Understanding Ag partner Allen Williams, Ph. D. helped one farm raise their pH from 5.7 in 2020 to 6.9 in 2023 by implementing adaptive grazing practices. No liming agent was utilized during that time. Many other incredible results were observed, which can be seen in Image 3 below.
Image 3: Otter Creek, NY soil data before and after implementing regenerative, adaptive grazing practices.
Understanding Ag consultant Morgan Hartman recalls a similar story working with the Hudson Mohawk Resource Conservation and Development Council in New York. The field in question had been hayed and/or bush hogged the previous 20+ years without anything being put back on it. Initial soil tests indicated a soil pH of 5.8 and recommended two tons/acre of high magnesium lime, along with 80 pounds of P and K. Instead of applying these expensive band-aids, Morgan decided to use the power of bale grazing to permanently fix the issue. Bale grazing is the practice of feeding hay on pasture, which benefits the land by adding large quantities of organic matter directly to the soil.
He suggested managers take 20 tons of late first cutting hay and place it on the field in the last week of August 2009, immediately following a graze that left about 6″ of residual. Cattle were introduced December 6, 2009 and removed April 6, 2010. Soil tests were conducted in May 2010 and found that soil pH had risen to 6.4, recommended no lime, no N, no K and only 40 pounds of P. Morgan added that they didn’t graze that field again until July 4, 2010 and that it’s still one of the more productive little plots on that farm.
Another example of the power of bale grazing was personally experienced by Understanding Ag consultant Jeremy Sweeten on his own farm in the Upper Peninsula of Michigan. Jeremy and his family purchased the heavy red clay farm in 2015, and soil tests indicated a high-magnesium soil with pH hovering around 5.5. The only liming product available in his area was high-magnesium lime, which made finding, purchasing and applying low-magnesium lime cost prohibitive. Jeremy and his family had to seek out alternative solutions. As 20th century physicist Ernest Rutherford said, “We haven’t got the money, so we’ll have to think.”
Instead, Jeremy improved his soils by managing the herd adaptively by utilizing paddocks, extending rest periods and feeding diverse hay bales. Soil pH became less of a hinderance to plant productivity especially once plant species diversity increased. The power of collaboration was on full display. Today, the county average for hay production in Jeremy’s neck of the woods is around 1.8 ton/acre/year. They now have pastures that triple that production. In addition, they also observe an increase in plant species diversity, soil organic matter levels and profitability.
Image 4: Pictures taken 2 years apart on Jeremy Sweeten’s farm showing the increased forage biomass production after 2 years of adaptive grazing practices.
Finally, if you thought this was purely an American phenomenon, you would be mistaken. English farmer Tom Burge, located in the southwestern county of Devon, has also utilized the power of adaptive grazing principles to eliminate the purchase of lime, fertilizer and chemical sprays on his pastureland. Many of his soils have pH around 5.5, similar to Jeremy’s, and range from dark-peat to deep-red clay. In addition, his farm receives more than 70-75 inches of rain annually, making outwintering livestock a real problem. Despite these challenges, Tom and his team have been bale grazing and rotating their Angus herd and sheep flock across the farm for the past five years. According to Tom, forage biomass has increased, soil pH is rising and he has increased his herd size, despite the warnings from others about the impending doom that would result from a reduction and elimination of lime and fertilizer.
What Now?
Good agricultural stewardship is about finding ways we can work within a given context to bring about more life, more balance and more positive relationships. There is a fine line between forcing our will on nature and giving an ecosystem a boost through human intervention. The exact location of this line is debated endlessly. Either way, soil pH getting off kilter unnaturally quickly is an ecosystem telling us we’re not managing it in a way that brings more life, more balance and more positive relationships. Nature is telling us something’s not right.
More times than not, the root cause is our desire (or industry demanding of farmers and ranchers) to produce more than the environment naturally would or should. We call that “Outproducing your environment,” and there are always negative compounding and cascading effects when an ecosystem is pushed past its limit. This is akin to the negative consequences that arise for a family, community or nation when money is slowly drawn out of a bank account quicker than it is replenished. There will eventually be a price to pay when money runs out, just as it is with the soil and the expensive amendments needed to amend pH while pursuing ever-increasing yields and maintaining overstocked pastures.
Conclusion
The emphasis placed on finding and curing root causes is a common theme running through regenerative agriculture and preventive healthcare. Soil pH is just one of many characteristics that farmers and ranchers battle whose root cause is likely self-inflicted. What if commercials for regenerative agriculture somehow made their way on TV? The narrator would end the ad saying something like, “Side effects may include building organic matter levels, growing soil aggregates, increasing soil microbial activity, more resilient plants, better water cycling, better nutrient cycling, soil pH mellowing…” Farm profitability and resilience in today’s world hinges on our ability to promote these positive compounding effects and to stop creating negative ones that require expensive inputs. Whether you experience money-saving positive compounding effects or expensive negative ones comes down to your management.
References
1https://www.nature.com/articles/s41467-020-18045-z
2https://soilhealthacademy.org/case-study/brandt-farms/
References
2 https://www.ndsu.edu/agriculture/extension/publications/what-soil-acidity
3 https://www.researchgate.net/publication/43518453_Role_of_organic_matter_in_alleviating_soil_acidity
5 https://archive.org/details/onuselimeinagri00johngoog/page/n6/mode/2up
7 https://en.wikipedia.org/wiki/Salting_the_earth
8 https://en.wikipedia.org/wiki/Yuma_Desalting_Plant

