4 Ecosystem Processes: Nutrient Cycle
Think Small
What do you think would happen if microbes that decompose stuff suddenly disappeared? Do we really need them? Do you think we would notice? The answer to the last two questions is a resounding YES! Without them, our world would rapidly pile up with feet of dead microbial, plant and animal material. We would eventually drown under a mountain of energy and nutrients because higher animals don’t have the right equipment to break down most of it into a usable form. Food, food everywhere, nor any bite to eat. Life as we know it would rapidly cease to exist. Lucky for us, we live in a world where we are vastly outnumbered by creatures that get their sustenance from roadkill, dead leaves, manure and other sources of nutrients we aren’t capable of utilizing. These microscopic marvels are the nexus between the living and the dead, resurrecting nutrients and energy back into the living. We certainly owe these unsung heroes a large debt of gratitude. At the very least, we should recognize their service to life on the planet.
In his influential book, Entangled Life, Merlin Sheldrake describes the moment he recognized the importance of decomposers. His father ran an experiment by cutting off the top of a clear plastic bottle and placing alternating layers of soil, sand and dead leaves inside the bottle. Next, they added earthworms and observed what happened. After a while, the distinct lines between the layers melted away. His father explained that the earthworms were not the only creatures disturbing the layers. Far from it. There were hoards of microscopic worms and smaller yet microbes that were consuming, mixing and stirring the layers. Sheldrake writes the following about the experiment, “Composers make pieces of music. These were the decomposers, who unmake pieces of life. Nothing could happen without them. This was such a useful idea. It was as if I’d been shown how to reverse, how to think backwards. Now there were arrows that pointed in both directions at once. Composers make; decomposers unmake. And unless decomposers unmake, there isn’t anything that the composers can make with. It was a thought that changed the way I understood the world.”1
While their importance may be unmatched, decomposers represent only a portion of a complex web of transformative interactions we call the Nutrient Cycle. Nutrients cycle efficiently in the presence of an abundant diversity of life. After all, who spreads fertilizer on the Amazon to get it to grow abundantly year-after-year? What about the African savanahs or the great diversity of life in the oceans? Before you say anything, I understand that we’ve got to feed the world. I understand that agriculture is unique compared to natural landscapes. What I hope to show is that regenerative agricultural systems can improve the health of a crop field or pasture, particularly in terms of the cycling of nutrients, to the point where the need for fertilizers is diminished or eliminated, just like in the forest, prairies and oceans. Imagine how great it would be to not worry about wars on other continents or OPEC dictating the price of fertilizer? Regenerative practitioners are proving this cut in fertilizer application is possible, all while producing the same or more calories and nutrient density per acre. It’s a big claim that demands big evidence, and I believe the evidence is there for those open to the concept.
The reality is that nutrients on most farms and ranches are cycling at a very unhealthy pace. Some nutrients are set in stone like Han Solo, while other nutrients are streaming away like the Millennium Falcon at lightspeed into surrounding streams, lakes and rivers. (Didn’t think you’d get a Star Wars reference in here, did you?) Most farms and ranches do not struggle with the absence of nutrients. It’s the cycling and availability of nutrients that is the issue. Getting the Nutrient Cycle running at a Goldilocks-level pace will make more nutrients available for plants and livestock, which will build healthier, more resilient and more profitable farms and ranches while maintaining or increasing production. The story of how this is possible reads more like a scientific textbook, not a fairy tale.

Nutrients: Atomic Legos
For many years, farmers and ranchers have been told to leave the thinking to the specialists. “Do as we say. Buy this, apply that.” Can you think of another career field where business owners are encouraged by so many people to leave their brain at the door? Agriculture is arguably the most scientific career field out there. It’s tied for first with many others, at the very least. The fact is that farmers and ranchers have so much to gain, financially and ecologically, from understanding the scientific principles at work in the natural systems they manage. Knowledge is what will help them wade through the constant arrival of miracle products and services that are marketed to solve their problems. Knowledge is what will allow them to become more self-reliant and resilient to factors outside of their control. Knowledge is what will make farms and ranches more profitable and more productive. I’m confident of this because the scientific principles that big companies use to make products can be understood by anyone. The same goes for advisers and their recommendations. There is no secret science they’re using that’s too hard for farmers and ranchers to comprehend. Farmers and ranchers are extremely smart people and I know they’re capable of understanding whatever they put their mind and their observational eyes to. For this reason, I believe the road to reducing fertilizer applications begins with a basic chemistry lesson.
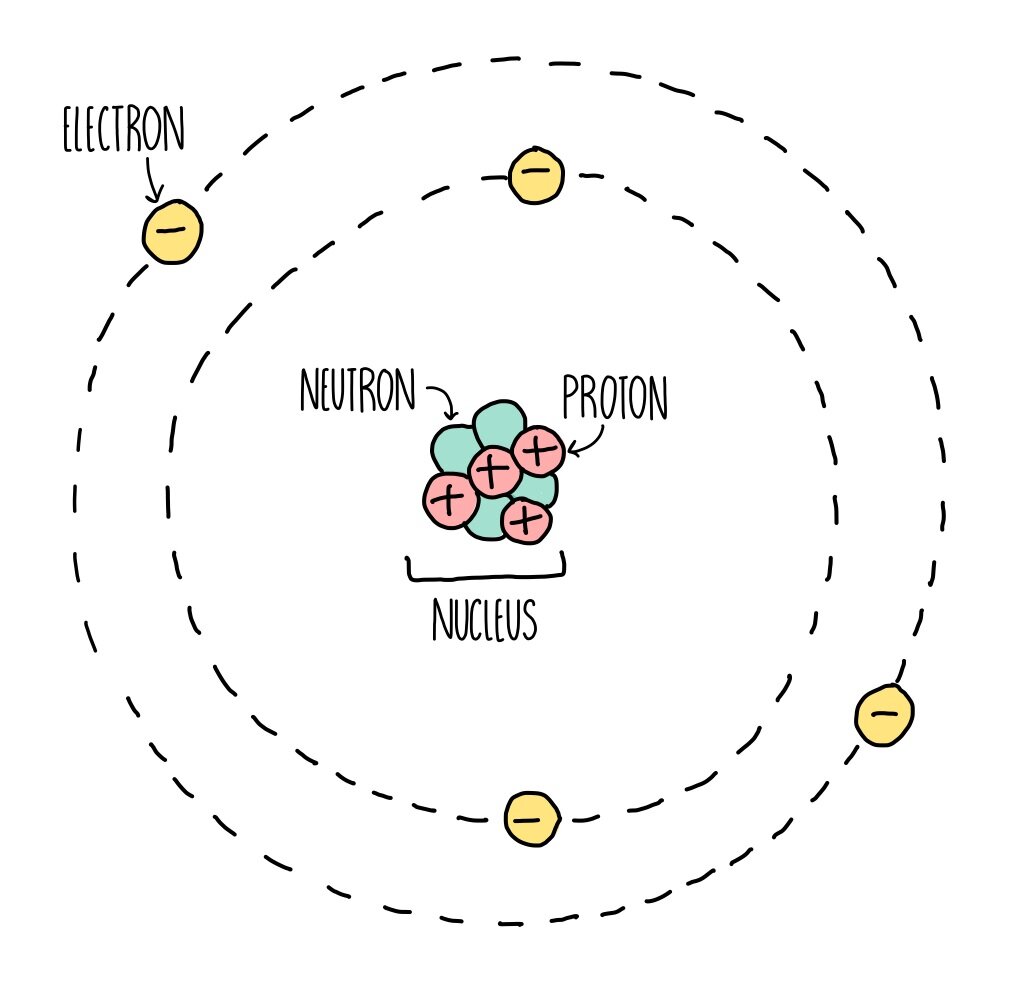
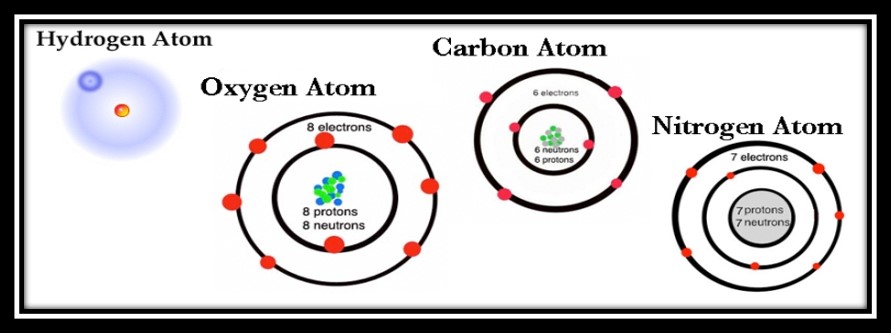
Science 101 tells us that animate and inanimate objects are made up of atoms, the building blocks of matter. Atoms are made up of a nucleus which contains positively-charged protons and neutral neutrons surrounded by negatively-charged electrons. The number of protons (+) and electrons (-) of an individual atom is the same, so the positive and negative charges cancel each other out. Among all atoms, the number of protons and electrons is what differentiates their identity. For example, Carbon has six protons and six electrons. Nitrogen has seven protons and seven electrons. Oxygen has eight protons and eight electrons. Congratulations, you now understand some basics of subatomic particles. You rock.
As you know, atoms connect with their neighbors to form complex structures. We wouldn’t be here if they didn’t. I find it helpful to think of atoms like Legos. They connect, get pulled apart, connect with something else, get pulled apart again, on and on again, all the while maintaining their identity, just like a blue Lego stays as a blue Lego no matter where it’s at. For this reason, the amount of oxygen, carbon and every type of atom on our mild-mannered planet remains the same. The only exception is if foreign space material crashes into the Earth or in extreme reactions, like the fission of uranium in bombs. Other than those rare exceptions, the only thing that changes with atoms is what they are attached to and where they are located!
Instead of raised edges and indentations like with Legos, atoms connect with other atoms through magnetism. They give or share negatively-charged electrons that are floating around the nucleus. Most atoms are stable when they have eight electrons in their outermost shell, except for the puny shell closest to the nucleus. It only likes to have two electrons. Check out the images below to see that the innermost shell is satisfied with two electrons, and then electrons begin to fill the bigger outer shell. Electrons on the outermost shell are given, taken and shared to make atoms more stable. Shared electrons count for both atoms!
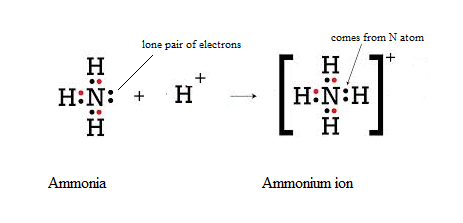
Let’s take a nitrogen atom, for example. Nitrogen just so happens to have five electrons in its outermost shell, which means it wants three additional electrons to be most stable. 5+3=8. A hydrogen atom is so small that it only contains one electron in its puny shell, so it wants to have or share one other electron to be stable. This means three hydrogen atoms sharing their electrons with one nitrogen atom makes everyone stable. We call this compound ammonia (a.k.a. NH3 because there is 1 nitrogen atom and 3 hydrogen atoms). Many farmers inject anhydrous ammonia into the soil to pump nitrogen into the system. Plants need nitrogen so this makes sense. However, plants don’t take up ammonia, so it needs to transform into a compound plants can use. What happens is that ammonia in the soil takes a hydrogen atom from water (H20) to share its electrons with. This makes ammonium (NH4 because we now have four Hydrogen atoms), which is a plant-available form of nitrogen. This small example illustrates an important point: chemistry is simply the movement of electrons. Nobel-winning biochemist Albert Szent-Gyorgi, best known for discovering Vitamin C, is quoted as saying, “Life is nothing but an electron looking for a place to rest.” Ag chemistry is no different. Chemicals or chemistry on the farm are just clever ways of utilizing the movement of electrons. Regenerative agriculture is all about creating a system where more of this movement of electrons is facilitated by living organisms on the farm or ranch, not by machines in factories that make costly inputs. As it turns out, living organisms are really efficient at moving electrons, while humans can be very wasteful, which costs the farmer or rancher big bucks. More on this later.
Atoms that are deemed important for the growth, survival and reproduction of living organisms are called “nutrients”. In agriculture, it’s widely cited that there are roughly 16 or 17 essential nutrients plants need in varying amounts. “Essential” means that a deficiency in the nutrient makes it impossible for the plant to complete its lifecycle. Essential nutrients needed in larger amounts are called “macronutrients”. The three nutrients needed in the largest amount are Nitrogen (N), Phosphorus (P) and Potassium (K). Scientists call N, P and K the “primary macronutrients”. Some universities are beginning to call Carbon (C), Oxygen (O) and Hydrogen (H) primary macronutrients as well.2 Calcium (Ca), Magnesium (Mg) and Sulfur (S) are also needed in large amounts, but not as large as NPK, so they are called the “secondary macronutrients”. Finally, certain nutrients are needed in tiny amounts, so they are called “micronutrients”. These include Chlorine (Cl), Zinc (Zn), Copper (Cu), Manganese (Mn), Iron (Fe), Boron (B), Molybdenum (Mo), Selenium (Se), Iodine (I) and Nickel (Ni). Some sources also list Silicon (Si), Sodium (Na) and Vanadium (V) as essential nutrients. Bored yet? Me too.
I personally find this arbitrary caste system of nutrients nice to have, but unhelpful in many ways. The focus becomes less about understanding the fascinating ways they behave and more about memorizing long lists of abstract facts of a topic that we’re discovering we know less about than we thought. Maybe that’s your thing. More power to ya, but most people don’t have the time or interest to memorize a zillion different facts about nutrition, whether we’re talking about crop nutrition, livestock nutrition or human nutrition. My contention is that this zillion-fact-strategy has led to a confused population of people that are told to rely on the experts for their fertilizer recommendations, feed rations and dietary guidelines, and we end up with something like the abysmal Food Guide Pyramid, which has since become the basically unheard of MyPlate campaign. The results from handing over the reins to these experts has been disappointing, to put it nicely. I also find the categorization of some nutrients as macro- or micro- to be unhelpful. These labels lead to the obligatory disclaimer in the beginning of any decent plant nutrition article that reads something like this: “micronutrients are just as important as macronutrients even though they’re needed in much smaller quantities.” This is nice, but ask most consultants and farmers which nutrients are most important to manage and the majority would respond with N, P and K. What happened to “micros are just as important”? There’s no judgement here, it’s just what has been taught, especially since World War II.
I believe farmers and ranchers would greatly benefit from a new curriculum on nutrition that leveled the playing field. Allow me to illustrate. Imagine a group of professionals are building a house. There are obviously going to be some materials that are needed in larger quantities by weight and volume than others, like concrete, wood, and drywall. Other materials like electric wires and appliances are needed in lesser quantities. As far as I know, nobody categorizes them into macro-materials and micro-materials. They’re all just necessary materials. In addition, they never pour the foundation, build the wooden frame, put up the drywall and leave thinking it’s a job well done. You might say that the “health” of the house is very poor at this stage. No food can be cooked, no clothes can be washed, there’s no wifi (the horror!) and burglars can break in with no locks on the doors. This is similar to what happens when nutrient management only focuses on certain nutrients. We produce crops or forage using fertilizer to make them look tall and green from the outside, but they’re really just a bunch of empty houses susceptible to weather, insect and pest pressure. You might say that the “health” of the plant is poor. We then train these unhealthy plants to funnel enough energy to produce fruits and grain in large quantities, but they also lack important contents. The “health” of the food is poor. Finally, livestock and humans consume these foods as the building material for their bodies. The result is that we become like a house built with only concrete, wood and drywall. The “health” of livestock and humans is poor. It’s very hard to debate the decline in American public health.4 There are various factors to this decline, but food quality is undeniably one of them. I’ll leave this topic for another day.
The N, P and K hegemony is slowly losing its grip on power, there’s no doubt about that. More fertilizer reps and university extension workers are discussing the importance of other nutrients, like calcium, sulfur, zinc and boron. The cynic out there might say they discovered new products to sell. The optimist might say this is a step in the right direction. Either way, the zillions of facts on nutrition science are mostly produced through a reductionist lens, and this is the crux of the issue. (Make sure to punch the “reductionism” square on your Regen Ag Bingo card if you haven’t already.) It’s true, reductionism has become a common target for regenerative speakers, but there is a very good reason for this. Living organisms are complex systems of endlessly interacting moving parts. This makes them really, really difficult to study in a laboratory, but it’s what science has attempted to do for the past century and a half, or more. This is like taking a car off the road, disassembling every single part, studying the individual parts and concluding what job each part had. In the process of disassembling it, they might, for example, shred the tires into tiny chunks of rubber, study the rubber and come to a false conclusion. Just as an aside, this is exactly why the leading theory on humus for roughly 150 years was incorrect. Soil treated with harsh laboratory chemicals created humic acid, fulvic acid and humin, which probably don’t exist naturally in the soil.5 Skip to minute 21 of this brilliant presentation by soil scientist Ray Weil to learn more.
Back to the car. Studying individual parts is useful in many cases, but society has relied almost exclusively on reductionistic conclusions in the past 150+ years. Trouble arises when we don’t pair these results with research that observes how whole systems operate. In other words, there is a lot to learn from observing the assembled car driving down the road, seeing where each part is located and discovering how the parts work together when the whole system is moving. This type of systems-based research is much more difficult, time-intensive and in many cases impossible. You can begin to see how the mainstream scientific community broke a plant down into a pile of atoms, discovered that nitrogen, phosphorus and potassium were found in large quantities, and deemed them the most important non-structural nutrients. Further experiments showed conclusive plant growth and yield increases with NPK fertilizer application, which reinforced the emphasis on the holy trinity of nutrients. Once again, I feel compelled to say that I have no judgement for those involved because reductionistic scientific research is a worthy endeavor and it has brought advancements to society of which I am forever grateful. The world was a different place not so long ago as communicable diseases still ravaged populations in the early 20th century, the world experienced the horrors of two world wars and people knew from first- or secondhand experience that Famine could gallop into town at any moment. As a result, yield was the top priority. Who could blame them? Since then, reductionist research, with its emphasis on NPK and plant breeding, has led to mind-boggling increases in food production over the past few decades, which has allowed the human population to skyrocket. I’m a fan of life, and I believe humans can improve the health of the planet, so I believe this is a good thing. However, we’re now discovering that there are many negative unintended consequences from the methods used to increase food production, particularly with regard to nutrient management. Acknowledging this fact does not denigrate the progress that our ancestors made. To close our eyes and plug our ears to the negative effects that have come as a result of modern agriculture is denigrating, both to ourselves and future generations.
Our job as land managers in today’s world is very different than what past generations had to do. In some ways, our objectives are much more difficult as we must increase food production on less land to feed a growing world population and do it in a way that improves the environment, all while turning a profit. On the other hand, we have increasingly better technology at our disposal which can help us work more efficiently and gather important information faster. For example, DNA analysis and computer modeling technology allow soil scientists to undertake desperately needed systems-level research in a timely and cost-effective manner. One day soon we’ll have a large database of systems-level research to pair with the mountain of reductionist research, especially as it concerns the Nutrient Cycle. This is encouraging news for a farmer or rancher looking to reduce their fertilizer bill! What’s more is there’s no need to wait until every single process is understood before producers begin taking advantage of natural nutrient cycling. Many producers have already proven it’s possible to understand the Nutrient Cycle, apply it to their own operation and decrease their need for applied nutrition, while raising abundant quantities of healthy crops and animals. It can be done. All it takes is convincing the living organisms on a farm or ranch to start moving electrons again and connect the right Legos at the right time.

Where the Wild Atoms Are
Learning the story of nutrient cycling is a necessary first step to memorizing the nutrients and how to manage them appropriately. Most of us were “taught” the latter without the former, which is an extremely difficult way for our brains to remember something. The story provides the substance for the facts to cling onto in our minds. I challenge you to sit down and read a bullet pointed list of facts on a topic you don’t know much about and try to recite the long list of facts you read once you’re done. This is a boring and ineffective way to learn something, but that’s the method we use to teach the rich topic of nutrition. Worst of all, this method fails to emphasize the fact that you are the leading character in the unfinished story of nutrient cycling that starts on the the farm or ranch and ends in the cells of human or animal consumers. Your management decisions dictate how the story is written. This may seem daunting, but remember, there are billions to trillions of supporting characters in your soil, air and water who are chomping at the bit to help you write the story.
One of the main goals of living organisms is to bring specific atoms in specific quantities from outside of their body to inside their body in a form they can use. Many atoms are then sent to become one of four basic compounds, or “macromolecules”: carbohydrates, proteins, fats and nucleic acids. Each will be described throughout the article. Because we don’t live in the vacuum of space, all of us living creatures on Earth are surrounded by atoms in the ground, water, air and bodies of other living organisms. Let’s start with rock and move our way up. Earth’s crust is the outermost solid layer and, believe it or not, is made up mostly of oxygen, followed by silicon, aluminum and iron. Most of us only think of oxygen as a gas, but oxygen combined with other atoms can be in a solid, liquid or gaseous form. The size and number of electrons in an oxygen atom make it quite the promiscuous character, as it wants to bond with various other atoms in the vicinity. In the crust, oxygen bonds create solids, such as with silicon dioxide (SiO2), the main constituent of sand. In fact, oxygen readily bonds with many metals and semimetals, not just silicon.
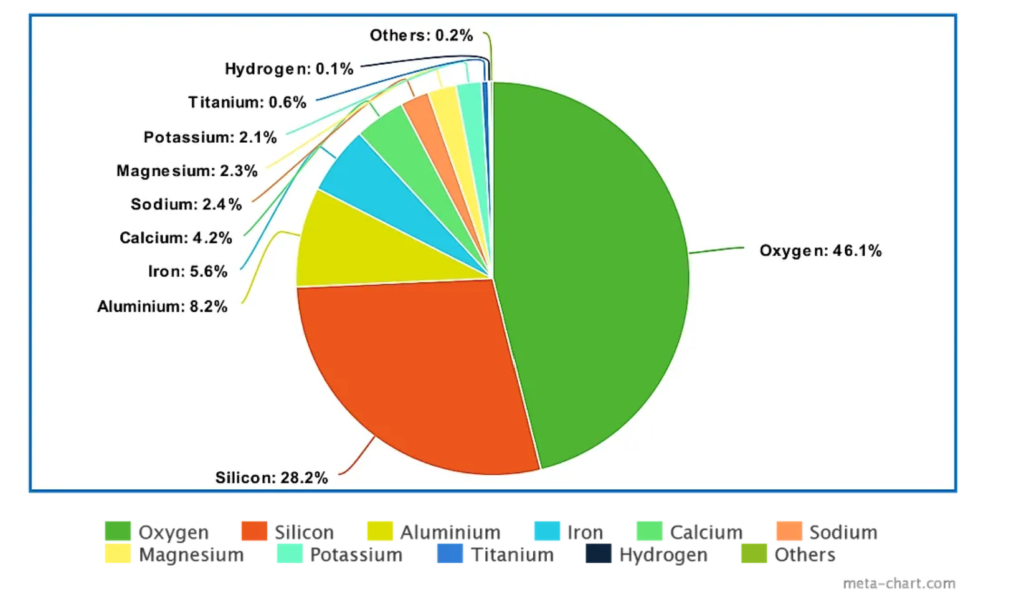
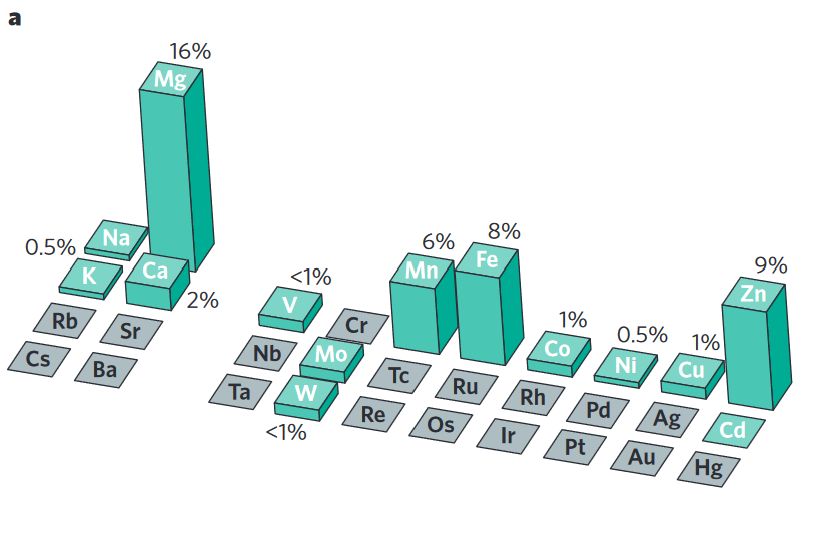
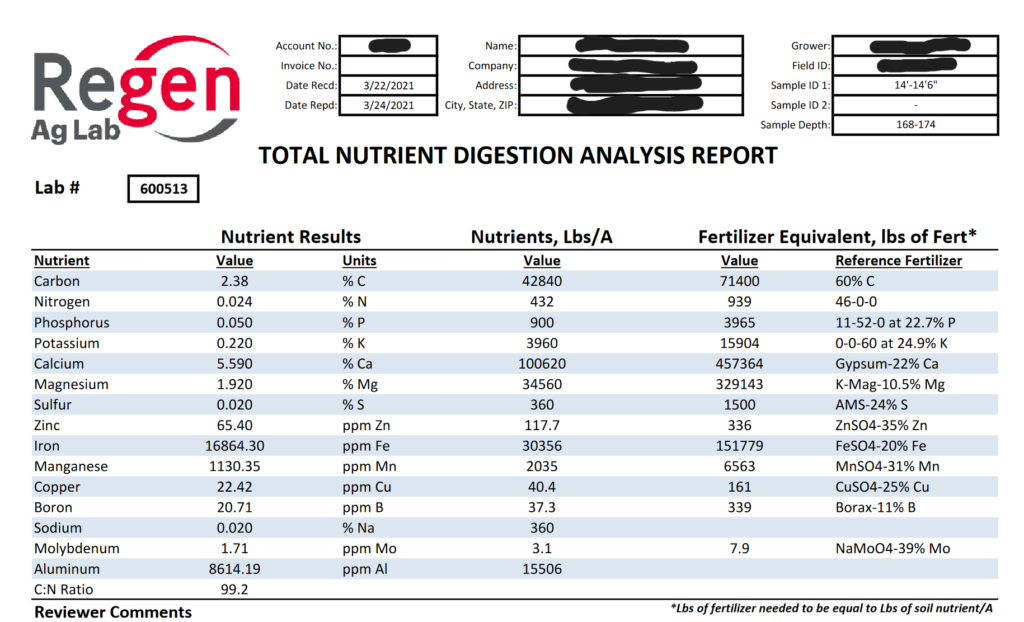
Atoms in rocks and minerals are held too tightly for living organisms to utilize until factors chisel atoms away from each other. These factors can be non-living (abiotic) such as rocks grinding against each other or water, physically, which can take hundreds or thousands of years. Another way to unlock these atoms is by living factors (biotic) such as organisms secreting strong chemicals that can dissolve rock into it’s constituent atoms for the purpose of consuming them and inserting them into their body. Pretty darn cool, if you ask me. This process can happen on the timescale of hours, greatly accelerating the cycling of atoms out of rock compared to weathering. The more biological activity in the soil, the more these atoms get chiseled away and can be used by living organisms. As you can see from the pie graph below, atoms important to plant growth, such as iron, calcium, magnesium and potassium, are found in the rocks and minerals of the Earth in significant quantities. Later, we will discuss the importance of the Total Nutrient Digestion (TND) soil test, which gives an idea of the full amount of various nutrients in the soil. Land managers are often surprised to learn that they have hundreds or even thousands of pounds of these nutrients in their soil. The challenge is to get this giant bucket of unavailable nutrients chiseled away and in the bodies of living organisms.
Oxygen also makes up a significant portion of the water that surrounds us, as it is one-third of the atoms and 90% of the weight of a water molecule (H2O; Two Hydrogen atoms and one Oxygen atom). For an in-depth look at water, check out the article on the Water Cycle if you haven’t already. Water is important to the Nutrient Cycle because it is considered the universal solvent, meaning it will magnetically bind with charged regions of other individual atoms or larger molecules. Water can also break break apart molecules into smaller pieces and carry them away, such as when sodium salt (NaCl) dissolves into a positively charged sodium atom (Na+) and a negatively charged chloride atom (Cl-). Similarly, water in the soil bonds with charged compounds and atoms that magnetically attach to water. This is important because plants can take up “water-soluble” nutrients like nitrate (NO3-) and ammonium (NH4+) through their roots. What water holds on to varies temporally and spatially. For example, hard water has a high concentration of calcium and magnesium dissolved in it, while purer water has much lower concentrations. Just like minerals build up on cups and silverware from hard water, soils with consistently high water tables result in a buildup of atoms, like sodium, left in the soil after water evaporates into the air.


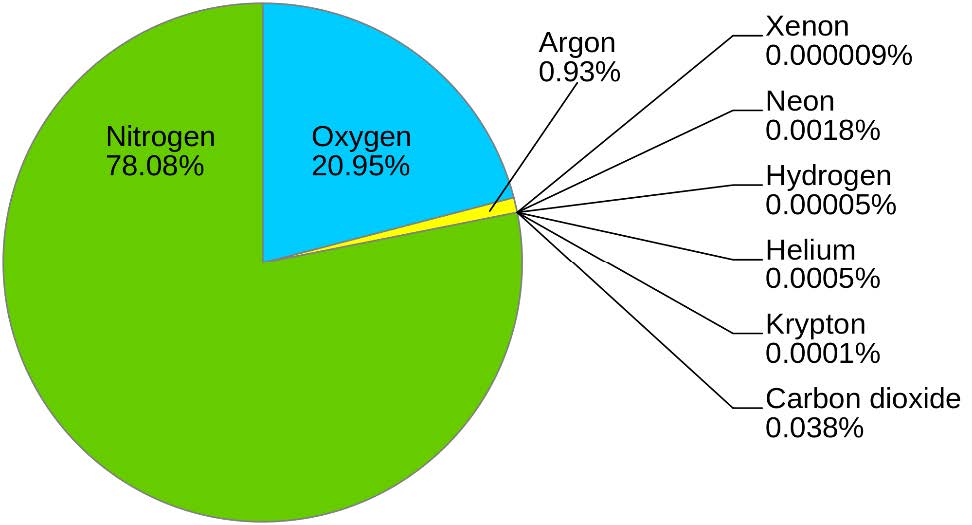
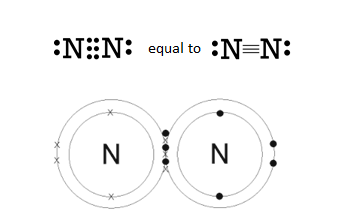
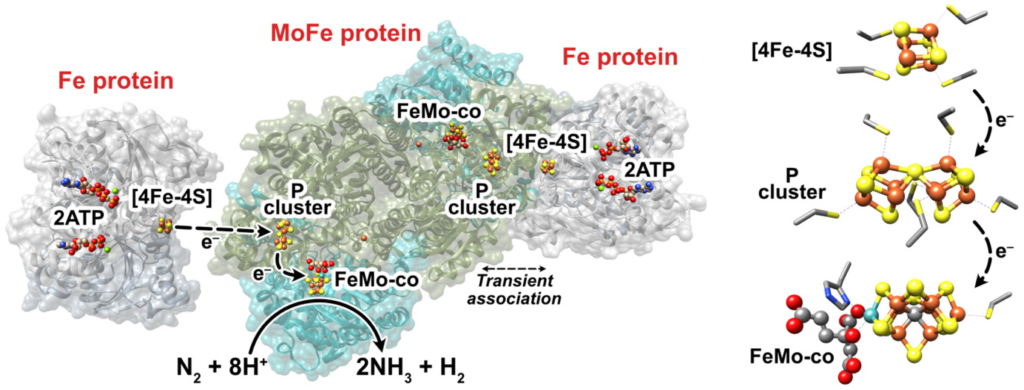
Moving upward, nitrogen makes up 78% of the air we breathe. We are literally bathing in nitrogen. In fact, over 30,000 tons (60,000,000 lbs) of nitrogen are above one acre at any time.6 Ironically, nitrogen is often the atom that limits plant growth and decreases yield potential. This is because nitrogen in the air is not available to living organisms, like the atoms held tightly in rocks and minerals. To understand why, recall that one nitrogen atom contains five electrons in its outermost shell, which means it wants three more to reach the magical, stable number of eight. As it turns out, two nitrogen atoms can share three of their own electrons to give both nitrogen atoms eight in their outer shell, making N2 gas. Remember, an electron shared between atoms counts for both atoms. This process creates a triple-bond between the two nitrogen atoms which requires an enormous amount of energy to shift electrons around and break it apart. Even so, many living organisms have devised strategies to gain energy from the separation of two nitrogen atoms. Legume-associated rhizobia bacteria are one such species. We will dig deeper into this process later.
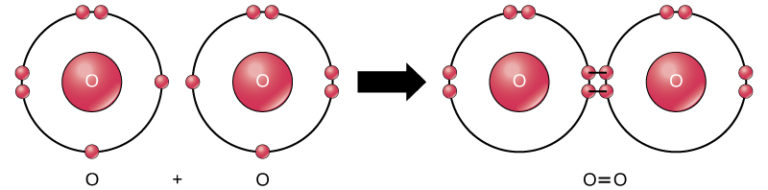
While oxygen is the most important atom in the air for us aerobes, it is a distant second in terms of abundance. The amount of oxygen atoms in the air rise or fall based on the quantity of photosynthesizing organisms on land and in the water. Photosynthesis splits water (H2O) into 2 hydrogen atoms and one oxygen atom. As you may recall, oxygen is promiscuous and doesn’t like to be alone. One oxygen atom has six electrons in its outer shell, meaning it wants two more to reach the magical, stable number of eight. Inside the plant, two oxygen atoms bump into each other and share two of their electrons in what’s called a double bond. This creates O2 gas in the air. The double bond of O2 requires less energy to break compared to the triple bond of N2 gas, just like a door with two locks is easier to open than a door with three locks. Air breathers like us take advantage of this fact and crack open O2 to make the energy we need to stay alive. This is called aerobic respiration and it’s the reason why animals, plants, fungi, bacteria and all aerobic organisms bring oxygen inside their bodies.
How Atoms Are Used
As you can see, the ingredients for life are all around us. Some atoms, like nitrogen and oxygen, are abundant, so it comes as no surprise that living organisms have them in abundance in their bodies. Others, like boron and zinc, are less abundant so they have a more specialized purpose, which we will get to. It’s up to living organisms to take atoms of all shapes and sizes from their surroundings and put them inside their bodies in the appropriate quantities at the right time. While there are patterns to the atomic composition of all organisms, no two bodies are the same. In addition, different strategies have developed over time to help organisms acquire atoms. To me, one of the most interesting topics in biology is discovering all of the different food sources that life on Earth takes advantage of to survive. To illustrate this point, let’s look at the atomic composition of living organisms, with an emphasis on plants.
The six most common atoms that make up living organisms on Earth are mnemonically known as CHNOPS (Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus and Sulfur). I remember it because it sounds like chin-ups. Plants fall under the category of living organisms, so they need CHNOPS in the highest amount. In fact, carbon, hydrogen and oxygen make up a whopping 94% of a plant by weight. Nitrogen is next in line at 3%. Luckily for them, these four types of atoms are abundant in most natural systems, which means plants have access to 97% of their weight right off the bat. Yes, even nitrogen.
Plants start the process by obtaining their carbon and oxygen as carbon dioxide (CO2), which they vacuum through the openings in their leaves. Hydrogen is transported to the leaf in the form of root-absorbed water (H2O). What happens next is a feat nearing the miraculous. Plants are able to create the right conditions which strip electrons from extremely stable water molecules that don’t want to give them up. This causes water to unzip into two free hydrogen atoms and one free oxygen atom, as previously mentioned. Next, the hard-earned electrons are welded onto another stable compound, CO2 ,which doesn’t want them. These extra electrons attract the free hydrogen atoms from the splitting of water. The result is that plants have created their own food in the form of a Carbo-Hydrate (C+H2O) known as glucose (C6H12O6). Dr. Brian Cox, British astrophysicist and professor of particle physics, writes in his book Forces of Nature, “This may seem unnecessarily complicated, but it probably isn’t. If you gave a chemical engineer the job of pulling electrons off water and putting them onto carbon dioxide, she’d probably laugh in your face.” And yet, this process of photosynthesis is exactly what plants do day in and day out to make the glucose they need to survive.
In summation, photosynthesis is responsible for capturing C, H and O, which end up becoming 94% of the weight of a plant. These atoms become the raw material that is used to build the structure of the plant, as well as the energy source to power all of the processes in a plant’s lifecycle (and eventually everything else!). The efficiency of photosynthesis is arguably the most important factor for plant health, plant production and yield. As it turns out, photosynthetic efficiency is much lower than theoretical values predict, with one study from MIT showing that plants convert light to sugars at 6% efficiency.7 This means there is a lot of room for improvement! Further research shows yields can be increased by more than 40% for various crops that were genetically modified to have higher photosynthetic efficiency.8 Whether you believe genetic modification is the way to go or not is besides the point. The point is that glucose (C6H12O6) production is one of the lowest hanging fruits we can focus on to improve the health and productivity of plants. This is why I personally dislike the the concept that C, H and O are not textbook “nutrients”. Even though a lot of other nutrients affect photosynthesis, and consequently C, H and O levels in the plant, I still believe the heart and soul of the process is left out by excluding them. The tide is slowly changing, however. For example, the “Plant Health Pyramid“™ developed by regenerative consultant John Kempf places improved photosynthetic efficiency at the base of the 4-part pyramid. Kempf teaches that plants with the capacity to produce more glucose and fuse a higher percentage of these glucoses together will be in a much higher state of health and productivity. Glucose production and fusing glucose both require the work of cellular helpers called enzymes. Enzymes fall under the category of protein, which leads us into the importance of the nitrogen atom.
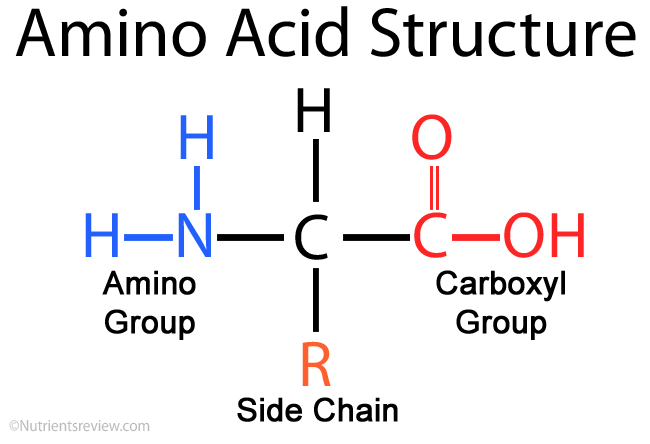
Proteins are among the most abundant molecules in the bodies of living organisms. While carbohydrates are fairly simple in that they form much of the structural skeleton and hold energy from the sun, proteins perform a near infinite amount of jobs. In fact, one cell may contain hundreds or thousands of proteins that all have different jobs. These jobs may be to support the structure of the cell, help cells move, act as signals so cells can communicate or facilitate the building or breaking of other molecules, which is what enzymes do. All proteins, no matter their job, are made up of units called amino acids that are linked together. Anytime you see “amine” or “amino”, think nitrogen. Every single amino acid contains a nitrogen, which is one reason why its demand is so high. Another reason is that nitrogen is also a key component of nucleic acids, the genetic compounds in living organisms. DNA (DeoxyriboNucleic Acid) is a nucleic acid which contains the blueprint for the production of proteins that make up living organisms. RNA (RiboNucleic Acid) is another nucleic acid, but its job is to transport and translate the information contained in DNA to sites that turn information into proteins. Lastly, nitrogen can be an important constituent of molecules that don’t fall under the umbrella of carbohydrates, proteins, nucleic acids or fats. One such molecule is chlorophyll. Each green-reflecting chlorophyll molecule contains four nitrogen atoms, which is why plants often green-up with nitrogen fertilizer applications. More chlorophyll molecules are produced, which increases the amount of green light from sunlight reflected back to your eye. Basically, nitrogen is at the heart of almost every single process happening in living organisms.
The final 3% of atoms that make up the weight of living organisms originate from rocks and minerals in the Earth’s crust. Abiotic (non-living) or biotic (living) factors chisel them away and make them available for uptake by a microbe, plant or animal. Once these atoms are released from minerals, they cycle in and out of living organisms as they live and die, get transported to locations via wind or water or become rock or minerals again by combining with other atoms.
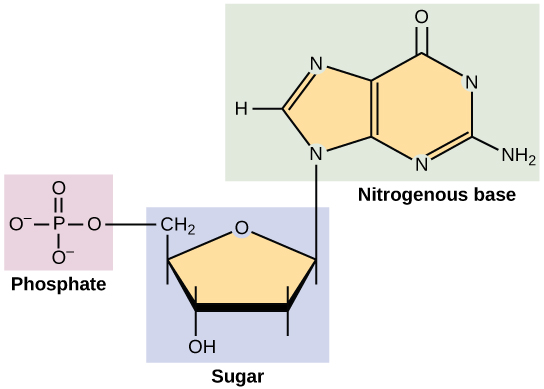
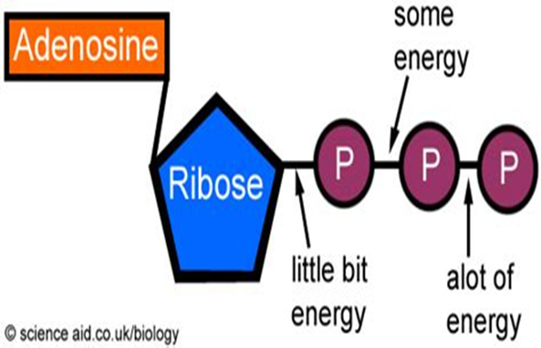
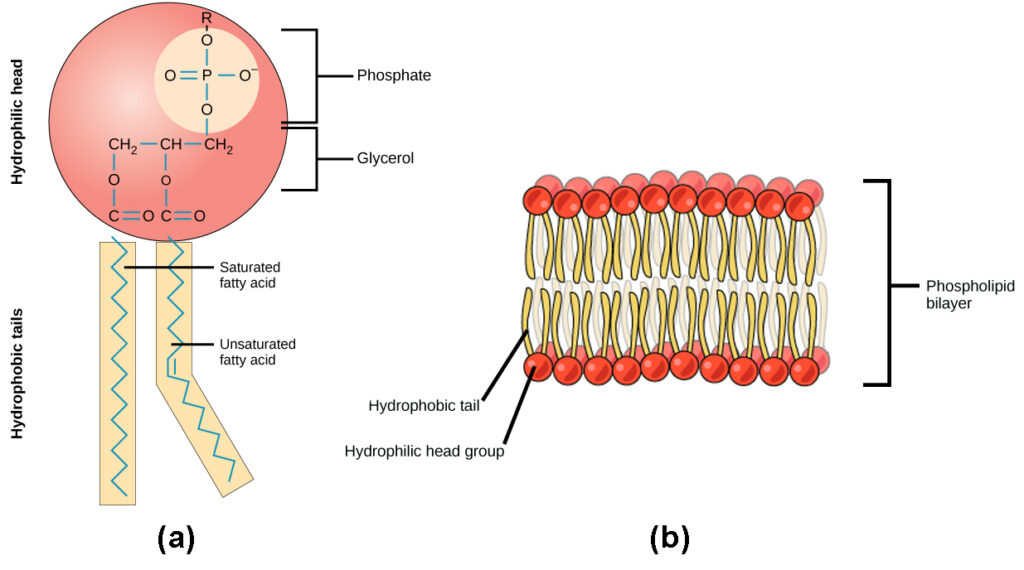
Phosphorus in the soil is notorious for quickly transforming back into a type of rock called apatite. (Fun fact: the hard outer surface of bones and teeth are made of calcium phosphate, a type of apatite.) This quick transformation back to rock happens to phosphorus that’s been chiseled away naturally and phosphorus that’s been applied as a fertilizer, with some estimates showing as little as 10–15% of phosphorus applied in fertilizers and manures is taken up by plants in the year of application.10 Despite this, phosphorus is a crucial atom for some of the most important processes in the bodies of living organisms. If you look at the image of DNA above, you will notice that phosphorus is the central atom for one of the basic units of DNA. The same goes for RNA. In addition, phosphorus plays an integral role in a molecule called Adenosine Triphosphate (a.k.a. ATP), which is considered the energy currency for all of life. Whether we’re talking about microbes, plants, livestock or humans, being alive and staying alive requires a lot of energy. ATP molecules are like mobile energy stations that can be transported to the site of an energy-intense process and release its energy to power said process. Check out the Energy Cycle article to get the lowdown on how ATP works. Lastly, phosphorus is an important component of the cell membranes of organisms. Cell membranes separate the cell from the surrounding environment, provide protection and allow transportation of items in and out of the cell, among many other jobs. It’s hard to overstate the importance of phosphorus.
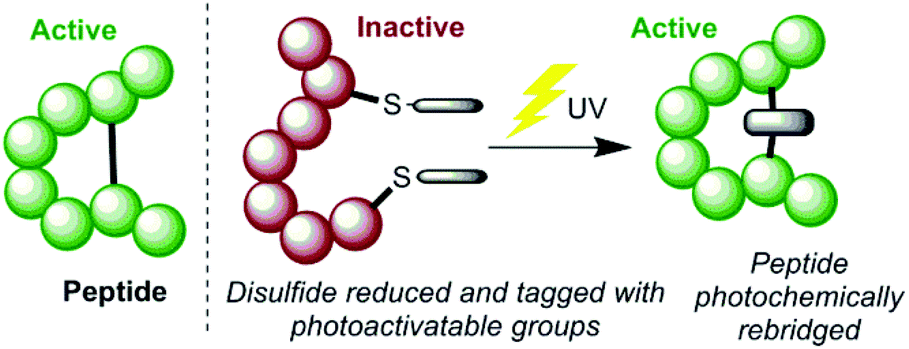
Rounding out the top six list of common atoms in a living organism is sulfur. This atom plays a central role in the proper functioning of proteins, as it allows the chain of amino acids to fold into the correct shape. In biology, function follows structure, so if a protein doesn’t have the right shape, it won’t work like it’s supposed to. Two important amino acids contain sulfur in their side chains: cysteine and methionine. Sulfur in cysteine will bond with sulfur from another cysteine to form “disulfide bridges”, which folds the protein in the appropriate spots. This can make the difference between an active protein and an inactive one, as the image below depicts. Methionine is also important as it is found in many proteins that uphold the structural integrity of the cell. Interestingly, sulfur is released during the burning of coal and other fossil fuels because these are old pieces of organic material whose sulfur is retained in their cells over time. Coal formed near brackish or saltwater environments contains higher levels of sulfur.11 Burning these ancient cells releases the sulfur as sulfur dioxide (SO2). Many farmers and ranchers used to receive annual sulfur amendments as the sulfur dioxide emissions dropped onto their land. Sulfur scrubbers and other environmental laws over the past decades have decreased the amount of sulfur dioxide in the air, and, consequently, the amount of free sulfur provided to the soil.
To recap the story so far, atoms are the basic building blocks of life. They function like Legos by connecting with other atoms to make complex molecules that can do different tasks. Bonds are formed by the giving, taking or sharing of negatively-charged electrons that orbit around the positively-charged nucleus. The movement of electrons is what makes life happen, essentially. Due to the number of electrons orbiting their nuclei, carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur (CHNOPS) atoms make up the vast majority of a plant, or any other living organism for that matter. They are the main constituents of the main molecules: carbohydrates (C,H,O), proteins (C, H, N, O, S), fats (C,H,O) and nucleic acids (C, H, N, O, P). Notice that carbon, hydrogen and oxygen are found in all four groups, which illustrates the incredible importance of these atoms to living organisms. Carbon, hydrogen, nitrogen and oxygen are found in the air and water prior to making up the body of a living organism. All other atoms originate from rocks or minerals in the Earth’s crust, including phosphorus and sulfur. Some atoms, like sulfur, can be found in gaseous forms in addition to mineral forms, but they originate from the mineral portion.
Living organisms utilize many other rock-originating atoms for various jobs in their bodies. These include potassium, magnesium, calcium and iron. There are plenty of great resources online to learn about the specific role of every plant nutrient, such as the thousands of free videos on “YouTube University” or these articles from Epic Gardening , Morning Chores and Alabama A&M Extension. Learning the different roles of each atom is always encouraged as more knowledge is better than less. Our adventure through the atoms will forgo the description of each remaining atom and go in a shiny, new direction to keep the general flow of the narrative moving along nicely.
One interesting fact about atoms (a.k.a. elements) is that most are metals. Incredibly, CHNOPS are not, as you can see in the periodic table of elements below. Is it a coincidence they make up the vast majority of living organisms? I think not. Their natural abundance and nonmetal characteristics make them the perfect candidates to create really stable molecules, kind of like the skeleton of a house. Now, it’s the job of the metal elements to make the house a home with their unique abilities. Metals currently recognized as essential to living organisms are Potassium (K), Calcium (Ca), Magnesium (Mg), Zinc (Zn), Copper (Cu), Manganese (Mn), Iron (Fe), Boron (B), Molybdenum (Mo) and Nickel (Ni). Some sources also list Silicon (Si), Sodium (Na) and Vanadium (V). Boron and silicon are technically “metalloids”, meaning they sort of behave like metals and sort of don’t. Chlorine (Cl) is the only essential atom after CHNOPS that is a nonmetal. Briefly, Chlorine is needed to break apart water molecules during photosynthesis and it aids in the operation of stomata.
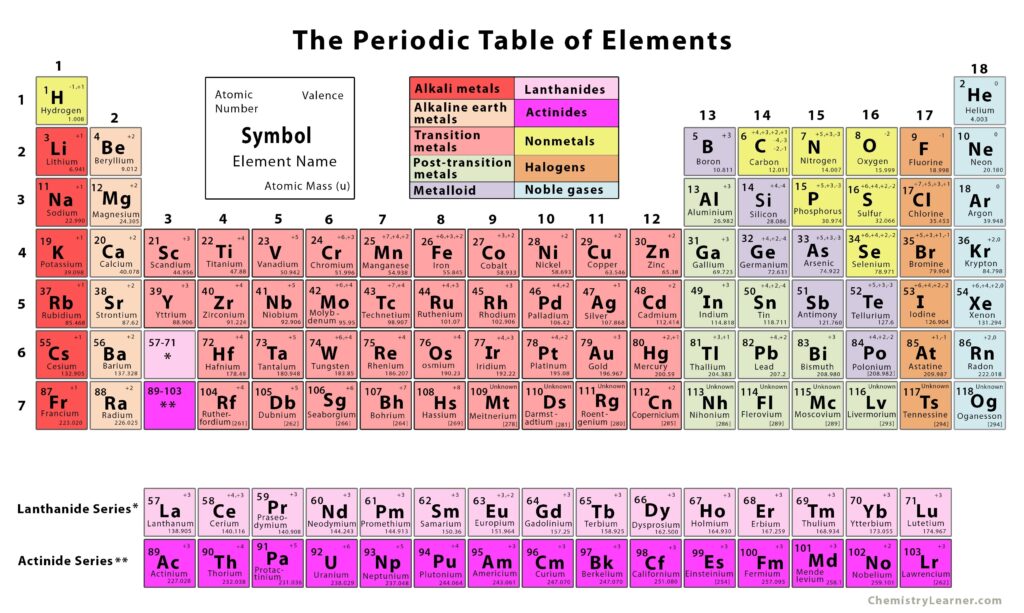
So, what makes an element a metal? Physical properties of metals include being shiny, conducting heat and electricity well, having a high melting point and being malleable, among others. These are tied closely to the chemical properties of metals. (Remember, Chemical = Chemistry, and chemistry is simply the movement of electrons.) These chemical properties include the fact that metals have 1-3 electrons in their outermost shells, they lose these electrons readily and they all react with oxygen to form oxides (think oxidized iron a.k.a. rust). The ability for metals to conduct electricity and move electrons is extremely important for living organisms. We wouldn’t be alive without them. Think of batteries in the TV remote or in the smoke alarm. Batteries power devices because there is a gradient of electrons that flow from high concentration to low concentration. Check out the article on the Energy Cycle for more details. As it turns out, living organisms utilize the flow of electrons as well, in what is called bioenergetics or bioelectricity (bio- means life or living things). For example, the nervous system of animals utilizes sodium and potassium atoms to create millivolt-level electrical impulses sent from the brain to the body and back. However, neurons aren’t required for bioelectricity, as the movement of sodium, calcium and potassium atoms gives our heart muscle fibers the gradient for an electric spark it needs to beat. EEGs are simply recordings of the electrical signals in the brain, while EKGs are recordings of the electrical signals in the heart. These neuronal and non-neuronal bioelectric signals are utilized by all living organisms on God’s green Earth, not just humans and animals. In fact, scientists have studied plant bioelectric signaling for over 100 years now.12
The point is that metals greatly influence the movement of electrons, and electrons are necessary for the use and production of energy in living organisms. In the house analogy, metals are like the electric wiring in the walls and batteries in appliances that allow countless processes to done. Copper, nickel, manganese and zinc are common atoms used in wires and batteries that living organisms also utilize to move electrons and conduct electricity in their bodies. Pretty cool, isn’t it? Proteins, being the workhorses that perform the near infinite amount of tasks required to keep living organisms functioning, are especially good at pairing with metals. To illustrate this, let’s look at one type of protein previously mentioned: the enzyme. Enzymes enable chemical reactions to happen, basically. Life-sustaining reactions that need molecules broken apart or fused together can take years, decades or even centuries to happen on their own. Enzymes enable these reactions to happen in seconds, and almost half of all enzymes require the presence of a metal to function properly.13
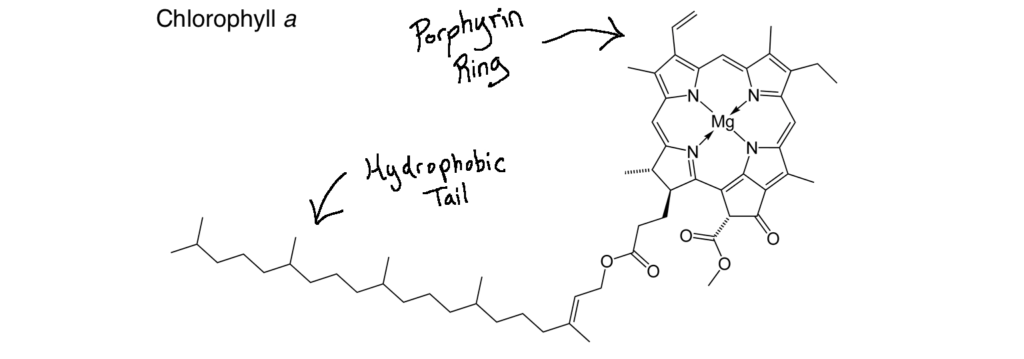
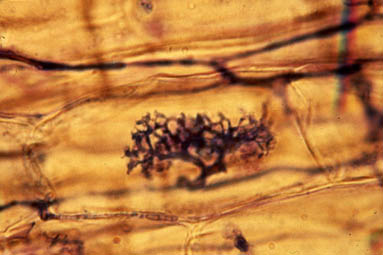
Nitrogenase is one such enzyme. (Anytime you see -ase at the end of a word, think enzyme). Anaerobic microbes, like rhizobia and azotobacter, deploy nitrogenase to break apart N2 molecules from the air and transform it into ammonia (NH3). Even though carbon, hydrogen and oxygen make up the majority of the nitrogenase enzyme, it will not be able to function without a tiny amount of iron and molybdenum atoms. Transformations all throughout the Nitrogen Cycle require enzymes at each step, such as ammonia monooxygenase, which requires copper and iron atoms, as well as hydroxylamine oxidoreductase which requires iron atoms as well. Inside of the plant, rubisco (Ribulose-1,5-bisphosphate carboxylase/oxygenase) is an important enzyme which catalyzes the first step in the fixation of carbon dioxide into glucose.14 Rubisco is the most abundant protein and enzyme on the planet. Its very presence allows for life on Earth to continue, and it won’t work without Magnesium atoms. Chlorophyll molecules, while not enzymes, also require a magnesium atom to function, as you can see in the “Chlorophyll a” image a few paragraphs up.
Other important uses for metals include a group of one of the most abundant proteins in biology called “Zinc fingers” (Honestly!), antioxidant enzymes dependent on manganese, and the various uses of potassium and calcium, among an infinitely long list of others. Novel biochemical discoveries are coming out nearly every day on the topic of molecular structures and their functions. This is an area of science to consider joining for all of you budding scientists out there! Just make sure to study how your specific molecule works in the real world as best you can 🙂
The goal of this section was to get across that the quantity of a given atom in a living organism doesn’t equate to importance because importance is subjective. Priority has been given to plant growth and fruit yield in the past hundred years or so, which is one reason why N, P and K became top dogs. Their applications create visible plant responses in the ways we’ve decided are most important. Scientists, farmers and ranchers did the best with the information they had, but we have the knowledge and the data today to prove that there’s much more to the story. Different types of atoms have different jobs that work together not only for growth, but for the health and longevity of the microbe, plant or animal. Keep this in mind when you further your education concerning the “macronutrients” and “micronutrients”. Another point worth noting is that the list of essential nutrients has grown over the decades, so it’s highly, highly unlikely that we have reached the pinnacle of understanding on plant nutrition. In my humble opinion, we should view the current list as a work-in-progress, not gospel truth. Therefore, an updated definition of essential nutrients may do a lot of good. UC Davis professor Patrick Brown and collaborators provide such an updated definition, which reads, “A mineral plant nutrient is an element which is essential or beneficial for plant growth and development or for the quality attributes of the plant or harvested product, of a given plant species, grown in its natural or cultivated environment. A plant nutrient may be considered essential if the life cycle of a diversity of plant species cannot be completed in the absence of the element. A plant nutrient may be considered beneficial if it does not meet the criteria of essentiality, but can be shown to benefit plant growth and development or the quality attributes of a plant or its harvested product.”15 It’s a step in the right direction, if nothing else.
Another huge step in the right direction is the growing acknowledgement that soil is not an inert substance that simply holds nutrients and water for plant roots. The fact is that atoms are continuously moving throughout the landscape via water, wind, temperature fluxes and, most importantly, biology. It’s hard for us macroscopic creatures to fully grasp, but the microscopic world runs the show, at least in terms of nutrient cycling. Many producers are figuring out that the microbes in their soil and on their plants can supply their crops or forage with all of the various atoms they need for vigorous growth, health and longevity. And, as we know, we are what we eat, so these health-promoting nutrients virtuously cycle upward into our livestock and eventually us. It all starts with our management of the microscopic herd.
Natural Nutrient Cycling in the Soil
The term “nutrients” will be used moving forward, but remember they are simply atoms giving and taking electrons, and they’re all very important. Now, the Nutrient Cycle is the wildly complex collective movement of every single shape-shifting nutrient throughout the environment. Our understanding of the natural cycling of nutrients has increased dramatically as our scientific tools have improved. We now know that many nutrients cycle together, while others antagonize each other. We know that some nutrients like to become solids, while others prefer to be liquid or gaseous. We also know that water, wind and other abiotic forces of nature move nutrients throughout the landscape, while living organisms consume and excrete others. Abiotic forces of nature tend to move the Nutrient Cycle along very, very slowly, while living biological entities move nutrients at light speed, comparatively.
To really understand the Nutrient Cycle and be able to manage it properly, there are a few foundational points to understand. In nature, nutrients can be part of the living or dead bodies of organisms, such as the ones that make up your body right now or the ones that make up dead plant residue in a field. These are called “organic nutrients” because they are part of Carbon-based molecules associated with organisms. In the science world, the term “organic” means it is only produced by living organisms and has carbon-hydrogen bonds, the hallmark bond shared by all living organisms. Methane (CH4) is an organic compound because it fits both criteria. Alternatively, “inorganic” nutrients are those not associated with organisms. These include the nutrients in rocks, soil and air that aren’t part of a carbon-based molecule, such as water (H2O), dinitrogen gas (N2), potassium (K+) and nitrate (NO3–). Even though it has a carbon atom, carbon dioxide gas (CO2) is considered inorganic because it can be produced by non-living entities, such as volcanoes, and it doesn’t have any carbon-hydrogen bonds. The carbon inside the carbon dioxide molecule transforms from an inorganic nutrient into an organic nutrient when a plant vacuums it in its leaf and fuses it with other carbon atoms to make glucose. The carbon goes from organic to inorganic again when molecules of glucose decompose. This is the case for all nutrients. For example, the nitrogen inside of a bacteria is in an organic form. When a predator consumes the bacteria and decomposes it in its digestive tract, the nitrogen may be stripped away from the larger carbon-based molecule it was attached to. One option is for the nitrogen to end up attached to four hydrogen atoms, in which case it is part of an ammonium molecule (NH4+). The nitrogen has thus transformed from being in an organic form to being in an inorganic form. The predator may then excrete this inorganic ammonium near the root, where the root can say “thank you very much” and slurp it up. This inorganic nitrogen could very well end up fusing to a larger carbon-based molecule and become organic nitrogen once again while inside the plant. This incessant shifting back and forth between organic and inorganic forms is happening all around us, as well as in our own bodies. It’s a natural part of what makes organisms and ecosystems alive. In contrast, nutrients on the Moon and Mars, and to a lesser extent in deserts, are stuck in one form or the other with little to no way to transform without living biological activity.
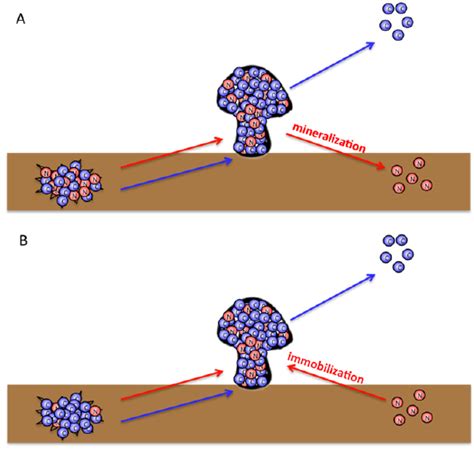
Two other terms that you will probably come across when studying nutrients are “immobilization” and “mineralization”. Immobilization refers to the process by which nutrients are taken in by organisms and made unavailable for other organisms to utilize. A common example is when soil microbes consume a lot of nitrogen in the soil, which “ties up nitrogen”, making the it immediately unavailable to plant roots. This can lead to nitrogen-deprived crops and one unhappy farmer. We immobilize nutrients when we eat food and make its contents part of our body. The same goes with plants or any other organism. It’s all immobilization. On the flip side, mineralization is the process by which nutrients are made available for other organisms to utilize them, such as the ammonium excreted from the predator in the previous paragraph. Natural systems do an excellent job of balancing these two processes. Immobilization keeps adequate nutrients in the system, while mineralization releases nutrients for new growth.
For the past 100+ years, conventional agronomy has taught that plant roots could only absorb nutrients in an inorganic and mineralized form. Search the web for “plant available forms of nutrients” today, and you will find numerous resources that have a list of “plant-available forms of nutrients”. Before or after the list, most resources state, “For a plant to absorb an element, it must be in a chemical form used by the plant and dissolved in the soil water (a.k.a. water-soluble).”16 This is the foundation for modern synthetic (a.k.a. man-made) fertilizer use. Synthetic fertilizer application is designed to place the inorganic, water-soluble, “plant-available form” of a nutrient in the soil where the plant root can absorb it. Simple enough… but that’s not what happens in reality.
First, it’s not true that plants can only absorb nutrients in an inorganic, water-soluble form. Let’s look at nitrogen. Plants (and mycorrhizal fungi) contain transport proteins in their cell membranes whose job it is to absorb amino acids, the organic building block of proteins.17 This saves the plant time and energy because plants have to fix absorbed nitrogen in nitrate (NO3–) and ammonium (NH4+) forms into an amino acid before it can be forged into a protein. Plants also absorb intact proteins, likely through endocytosis.18 If they don’t want to consume the whole protein, they can break up proteins in the soil by exuding proteolytic (proteo- protein; lytic- breaking apart) enzymes. These enzymes break apart proteins into smaller chains of amino acids or individual amino acids that the plant can then choose to absorb. Endocytosis and proteolytic enzymes are very important because nitrogen primarily occurs as protein in natural soil ecosystems.18 This is a huge reservoir of nitrogen, so you can imagine why plants have methods of utilizing it! (Side Note: None of the nitrogen in this huge reservoir will be counted in a standard soil test because it’s not in an inorganic, water-soluble form. However, we just learned that organic nitrogen can and will be utilized by the plant, so this inevitably leads to overestimates of fertilizer need.) We also know that plants absorb nutrients in organic form because organic compounds like citric acid are utilized in projects that clean up dangerous heavy metals from a contaminated soil.19 Nutrients like lead, arsenic and cadmium bind to the citric acid compound and plants take up the Heavy Metal/Citric Acid organic complex, thus reducing the amount of heavy metal contamination in the soil. This would not be possible if plants could not absorb nutrients in an organic form. Lastly, plant absorption of herbicides and whole microbes (a.k.a. rhizophagy) are further proof that they absorb larger, carbon-based organic compounds.
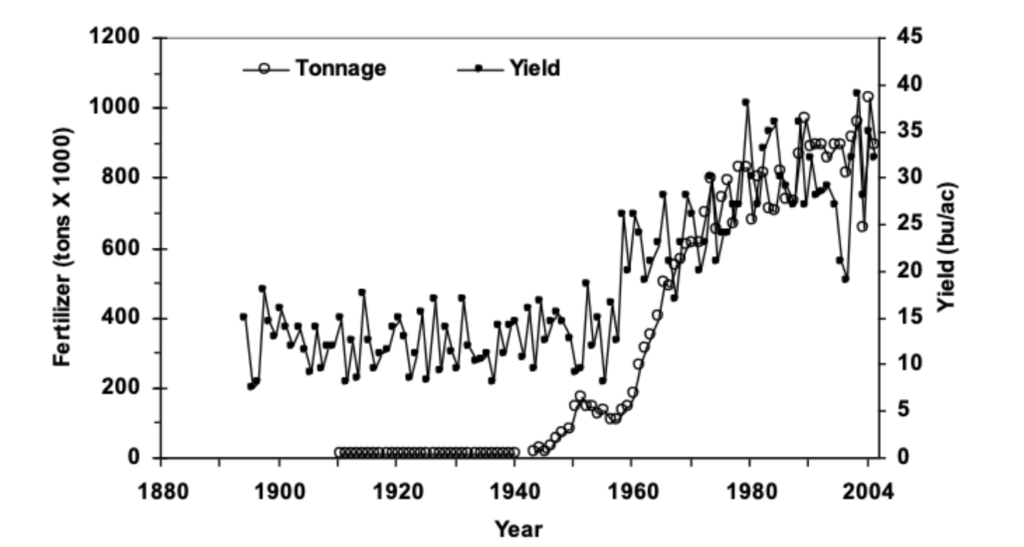
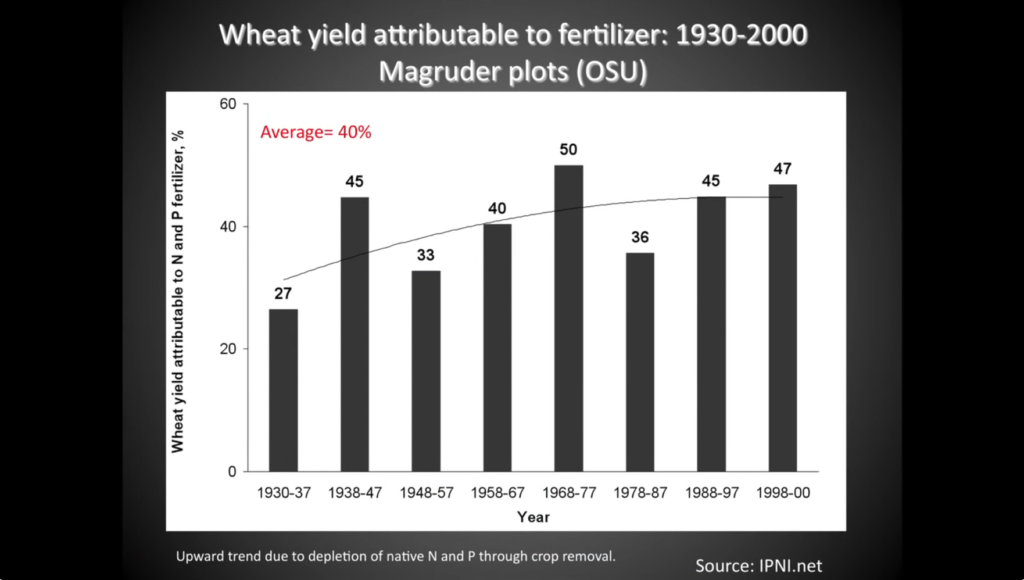
Second, nutrients from synthetic fertilizers don’t feed plants. Most nutrients are acquired from the natural Nutrient Cycle, even after fertilizer applications are made. The 15th edition of The Nature and Properties of Soil textbook writes, “A common myth about fertilizers suggests that inorganic fertilizers applied to soil directly feed the plant, and that therefore the biological cycling of nutrients are of little consequence where inorganic fertilizers are used. The reality is that nutrients added by normal application of fertilizers, whether organic or inorganic, are incorporated into the complex soil nutrient cycles, and that relatively little of the fertilizer nutrient (from 10 to 60%) actually winds up in the plant being fertilized during the year of application. Even when the application of fertilizer greatly increases both plant growth and nutrient uptake, the fertilizer stimulates increased cycling of the nutrients, and the nutrient ions taken up by the plant come largely from various pools in the soil and not directly from the fertilizer. For example, some of the added N may go to satisfy the needs of microorganisms, preventing them from competing with plants for other pools of N.” Phosphorus absorption from fertilizers is particularly woeful as more than 80% of applied P is immediately made unavailable after application.20 How many farmers and ranchers are told by their fertilizer salespeople or advisers that 40-90% of the fertilizer they purchase won’t end up in their plants? That’s a hefty chunk of change! This isn’t to say fertilizers don’t work and they should be ditched. The image below shows a pretty close relationship between fertilizer use and wheat yield in Oklahoma from 1890-2004. Of course, fertilizers aren’t the only factor in this rise in yield over time, but they certainly play a role. In any case, it’s important to understand that the majority of nutrients absorbed by a plant run through the natural Nutrient Cycle before reaching the roots. Data from the third longest-running agricultural research project in the United States corroborates this claim. Their experiment found that around 40% of wheat yields from 1930-2000 were attributable to fertilizer nutrients. 40% is a big boost and that’s great, but a few questions might come to mind:
- Where exactly does the other 60% of nutrients absorbed by plants come from?
- Can this number be higher so we have to purchase less fertilizer?
- How do plants in the wild grow so well without synthetic fertilizers?
- Do unused nutrients build up in the soil over time, and do farmers and ranchers stop needing fertilizer eventually? (Bring a stopwatch with you to time how long it takes a farmer or rancher to stop laughing if you ask them this question.)
Underground Herd
Nutrients transform, translocate and become available to plants via abiotic (non-living) and biotic (living) factors. Abiotic factors include the weathering of rocks by carbonic acid (formed when carbon dioxide mixes with water), glaciers, lightning, volcanic activity, pressure and others. Some of these pathways move nutrients very quickly, but most of them take decades or longer to make appreciable contributions to the reservoir of plant-available nutrients. Vigorous biotic activity, on the other hand, cycles nutrients on a timescale of minutes, days and weeks. As a matter of fact, this is one of the fundamental characteristics of life. Creatures constantly need inputs of energy and raw material to build and maintain cellular constituents. In other words, we all need a constant stream of food to grow and replace the waste we excrete along the way! Every living organism on the planet acquires energy and raw material by 1) producing chemicals and proteins that allow them to utilize the resources around them, 2) competing for these resources with nearby organisms and 3) teaming up with other organisms when it is beneficial to do so. Soil scientist Dr. Elaine Ingham calls the sum total of these relationships the “Soil Food Web“. This imagery is more appropriate than a food chain, because the biotic cycling of nutrients is circular. Plants, animals and other organisms at the “top” are eventually broken down by decomposers at the “bottom”, thus bridging the two ends of the spectrum. Energy and nutrients inside of microbes will eventually become food for something else either when they are eaten or when something eats their energy- and nutrient-rich waste and by-products. Imagine a bacteria that has been eaten and digested by a predatory protozoa. Some of the bacteria is used as energy and building material for the protozoa, while other parts are excreted as waste where some other organism, like a plant, can make use of this micro-manure. This consumption-waste-consumption-waste web of interactions is ubiquitous, and it’s a process that is ecologically and economically wise to prioritize. The key to managing this process efficiently is to provide a constant input of food into the soil system for the underground herd. Otherwise, hungry microbes will consume organic matter in the soil quicker than it is replenished. Adding compost and manure are good options when done appropriately, but the most effective method of feeding soil biology is by growing a plant. It sounds simple, but it’s true. In regen ag-speak, this is called “maintaining a living root in the soil for as long as possible throughout the year“. Living plants are the bottleneck that transfer solar energy to the soil. This empowers living organism to do things, not least of which is a furious cycling of nutrients that benefits everyone, especially plants!
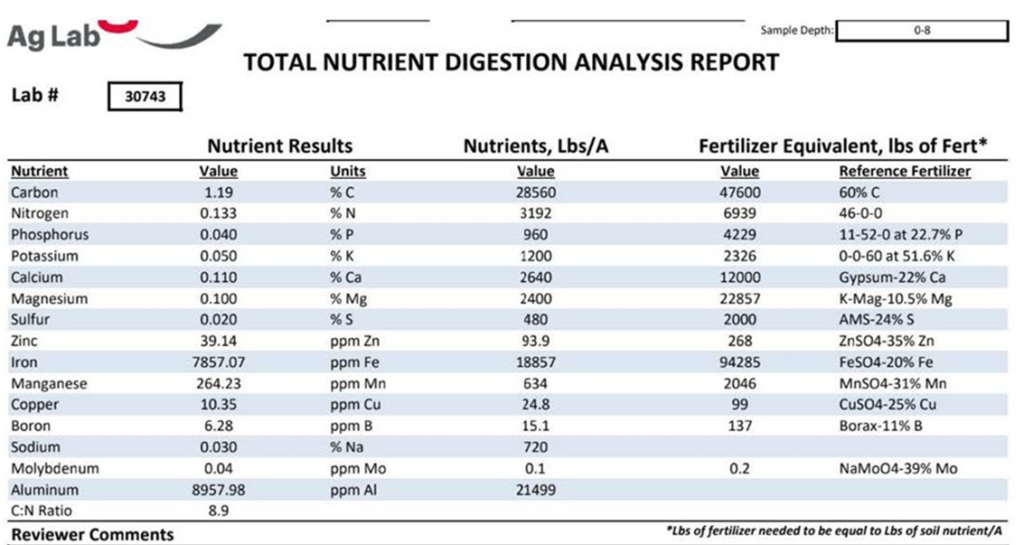
But wait, won’t increasing the number of plants in a field or pasture speed up the mining of nutrients out of the soil?? This concept of nutrient mining is a core tenet of conventional nutrient management and has been for decades. It makes sense with the testing and technology that was available in the past. It also makes sense because we do happen to live in a finite world with finite resources. Matter and energy cannot be created or destroyed, after all. However, advances in soil science and soil microbiology are showing that past technology only gave us insight into a fraction of the nutrients in the soil profile. Tests were designed to see the inorganic, water-soluble nutrients that were available to the plant at the moment the soil test was taken. As it turns out, nutrients that fit this description are a tiny fraction of the overall pool of nutrients in a landscape. Conventional soil tests can neither detect this giant reservoir of total nutrients nor calculate the quantity of nutrients that will be transformed into forms that plants can utilize throughout the growing season. The classic example is nitrogen. Many conventional soil tests calculate the quantity of nitrogen in one specific form: nitrate (NO3-). Even so, this calculation is not usually offered in a base package and is deemed an “optional test“. This is an issue when calculating N fertilizer needs because nitrogen is found in many different forms, not just nitrate. This includes the 30,000+ tons of N2 gas above every acre. We also know that biology is incessantly transforming and translocating nutrients throughout a landscape, including the diazotrophs that can turn some of the inert 30,000+ tons of N2 gas into forms that plants can utilize. Therefore, it’s important to understand that soil tests are snapshots in time of nutrient availability. Enormous changes will occur throughout a growing season, including a natural drop in soil pH as roots and microbes release organic acids during the growing season.21 All of this is way above a conventional soil test’s paygrade, but it’s all really important, and it’s all really happening in crop field and pasture ecosystems. For this reason, the Total Nutrient Digestion test has been developed to allow producers to see the total reservoir of nutrients in a soil profile. These data points are extremely useful when paired with PLFA and Haney soil tests, which measure biological populations and their activity. The PLFA and Haney tests give producers a better idea of how many nutrients will be turned into forms the plant can use as the season progresses. Once again, land managers need to start thinking small if they want to save big on their fertilizer bill.
To understand how such small creatures can have such a huge impact on nutrient cycling, we have to think about life from their point of view, not ours. First, microbes are found pretty much everywhere because of their ability to reproduce at a very rapid pace. In fact, bacteria reproduce every 20 minutes and viruses reproduce even quicker.22 Humans, on the other hand, reproduce a time or two by age 30, roughly speaking. Not only does this pace increase microbial population size quickly, it also increases their chances of surviving in ever-changing conditions because quick reproduction raises the chances of beneficial random mutation. In addition, epigenetic regulation helps individual microbes adapt to changing conditions by turning genes on and off as needed. Horizontal gene transfer and quorum sensing allow microbes to adapt as a group and pass on helpful genetic information to their peers without sexual relations, à la Bill Clinton. Therefore, it’s no surprise that microbes have learned over time how to utilize the available resources in virtually every location on Earth, both individually and as a group.
So, if microbes have conquered harsh environments like mountaintops and underwater thermals vents, imagine how well they would do in a temperate, moist environment with abundant resources. The soil, as it turns out, is just such a paradise where microbial populations thrive. In fact, the soil is likely the most biologically active location on Earth, with one estimate showing nearly 60% of all life on Earth lives in the soil.23 Microbes comprise the vast majority of this explosion of life and diversity. This diversity is crucial because individual species find their niche by extracting energy and raw material from specific materials. The interactions between resources and organisms that use these resources keep the system moving along competitively and collaboratively. For example, diazotrophic microbes gain nitrogen for their bodies by taking nitrogen gas (N2) in the air and turning it into available forms that life can utilize, i.e. ammonia (NH3) or ammonium (NH4). This nitrogen also becomes available for other microbes who might make a living by moving phosphorus out of rock and into forms that life can use24. The same goes for potassium25, sulfur26, calcium27, silicon28 and every other nutrient a microbe can get its enzymes on. Living organisms then consume these nutrients and make them part of their bodies. These microbes die or get eaten by predators, and the cycling of nutrients is off and running. In essence, this means that microbes naturally fertilize the soil with their ability to transform nutrients from forms that plants can’t use into forms they can. Even better news is that most, if not all, nutrients are found in abundant quantities in the soil. Yes, even P and K are abundant! The authors of reference #26 succinctly write that phosphorus is “abundant in soils” and the authors of #27 comment that, “Naturally, potassium (K) is an abundant element present in the soil but in an inaccessible form”. They said it, not me!
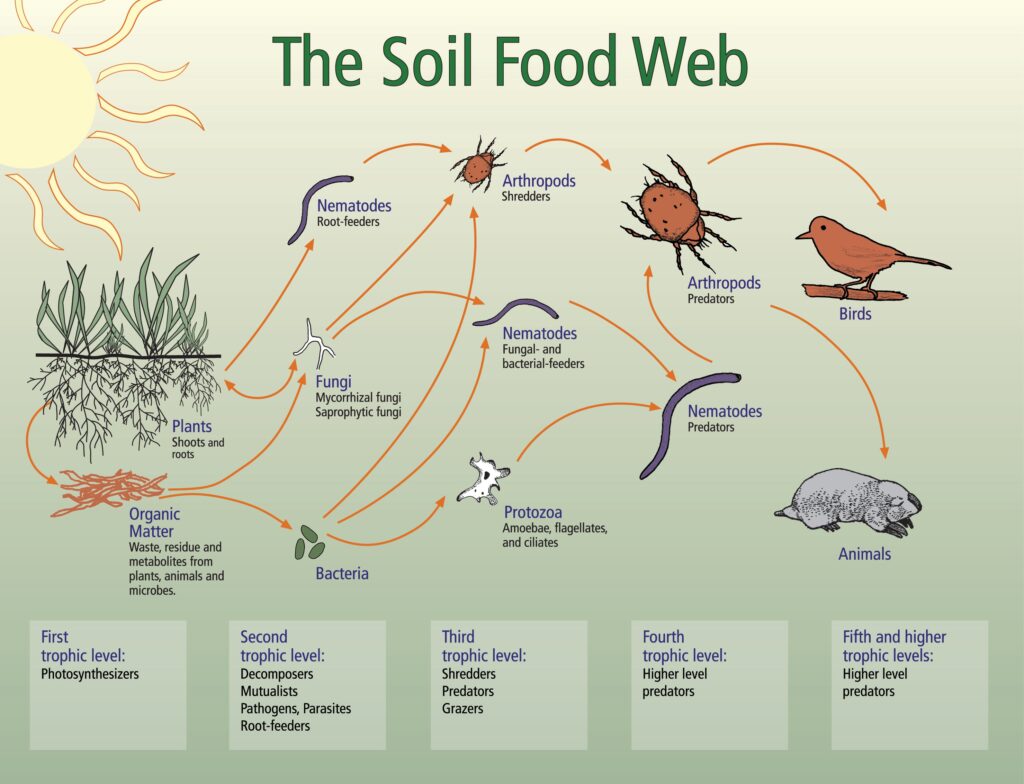
It’s no surprise, then, that plants have developed a cozy relationship with the ever-present, nutrient-cycling microbial communities. Microbes live on the leaves, stems, roots and interior of plants. We call the zone of interactions in the aboveground portion of plants the “phyllosphere” and the belowground zone of interactions are called the “rhizosphere“. Microbes that enter inside the plant are called “endophytes“. The vast majority of bacteria, archaea, fungi, nematodes and protozoa associated with plants are either neutral or beneficial in terms of nutrient acquisition. This is true even for the phyllosphere as some bacteria living on the leaves of plants are capable of fixing nitrogen from the air into forms that plants can use, just like the bacteria that partner up with legumes.29 Who knew! Unfortunately, the phyllosphere is the least studied of all the plant-microbe relationships, so more research needs to be done to help farmers and ranchers maximize phyllosphere nutrient cycling, especially as foliar feeding becomes a more popular option of fertilization. For this reason, nutrient cycling via rhizospheric and endophytic relationships will be the focus moving forward.
Technically, the rhizosphere is the 2-3 mm zone surrounding the root. This area is teeming with life, death, consumption, excretion, fighting, collaboration and transferring of information. In fact, the rhizosphere is arguably the most complex ecosytem on the planet.30 How many farmers and ranchers know that such complexity and richness exists right beneath their feet? Not many, so hopefully the word gets out. Many regenerative producers report a sense of awe and wonder upon learning about this underground frenzy of life, which inevitably leads to a sense of responsibility to care for these organisms with as much passion as they care for their crops and livestock. This shift in perspective is a winning long-term strategy both ecologically and economically for a farming or ranching operation.
As was previously mentioned, plant roots are the best food source to revive and maintain soil microbial populations. Plant roots themselves affect the nutritional profile of the rhizosphere because they absorb nutrients from the soil. In fact, roots are designed to absorb nutrients in this zone efficiently, so easily absorbed forms of nutrients become depleted rather quickly. Being the chemistry wizards that they are, plants also produce and exude compounds outward into the soil to help make more nutrients available, such as organic acids that catalyze the release of nutrients from nearby soil minerals and organic matter. Unfortunately, nutrient absorption by roots alone is not enough to support a growing plant. Plants greatly increase their access to nutrients by teaming up with the trillions of soil microbes that work tirelessly to bring nutrients into the realm of the living. Microbes, like any good worker, happily oblige as long as they get paid for their services. Plants agree, and soil microbes inhabit the rhizosphere at very high populations, with some figures showing that there are 10–50 times more bacteria and 5–10 times more fungi in the rhizosphere compared to soil away from the roots.31 Plant roots feed microbes passively and actively, as well as provide a friendly environment for their proliferation. Passively, microbes consume sloughed off root cells and the mucous-like substance emitted by roots that reduces friction as root tips grow through the soil. Actively, plants exude organic compounds to attract microbes to the roots. Once there, plants exude specific compounds which favor the growth of certain species depending on nutritional needs throughout its life cycle.32 In essence, plants give energy to soil microbes and receive nutrients in return. It’s a beautiful exchange because plants are expert photosynthesizers, but not great soil miners. One small example involves certain bacterial strains of Bacillus, Pseudomonas, Burkholderia and Acinetobacter, which increase availability and uptake of micronutrients such as zinc and iron for mung bean, rice and corn.33 Another more common example is the relationship between nitrogen-fixers and plants, including legumes and rhizobia, as well as free-living diazotrophs like Azotobacter, Clostridium and Azospirillum that associate with various species of plants. Interestingly, many producers observe fewer nodules on legumes as soil health improves because legumes are relying less on rhizobia for their nitrogen needs and more on other sources of available nitrogen, including the free-living fixers. Plants also associate with various phosphate-solubilizing bacteria that make phosphorus more available for uptake. This is helpful but sometimes the bacteria are outside of the rhizosphere, so there needs to be a middle man to deliver nutrients to the plant. Enter fungi.
Soil fungi make up only 10-30% of rhizosphere by population, but they lead the pack in terms of mass when the soil is “healthy” and undisturbed. Tillage is especially harmful because their long, spindly bodies cannot survive tillage events like a teeny, tiny bacteria or archaea can. Now, in the intro, we learned about decomposer fungi and their role in cycling nutrients. Fungi (or bacteria) that break down the bodies of dead organisms are called “saprophytes“. Even though it’s an unsexy job, saprophytic fungi are arguably the most important regulators of nutrient cycling in the soil. Without them, nutrients would have to be made available again via abiotic forces, which can take decades or longer. Instead, nutrients like nitrogen, phosphorus, potassium, calcium and the rest are made available to life in minutes and days after an organism dies. White rot fungi are a great example as they are one of the only species that has cracked the code to unlocking and releasing nutrients from material that is very hard to break down, like wood. Lignin is the compound that makes wood “woody” and contains enormous amounts of carbon, which white rot fungi bring back into living circulation decades and centuries quicker than it may have otherwise.34
Fungi don’t just decompose dead organic matter. Another major class of soil fungi are called “mycorrhizal fungi“. Mycorrhizal describes the relationship between plant roots and fungi (the prefix “myco-” means fungus and “rhiz-” means root). Plants with roots that associate with fungi are mycorrhizal plants, while fungi that associate with roots are mycorrhizal fungi. It’s estimated that 80-90% of all plants are mycorrhizal and, depending on who you ask, this relationship may date back more than 450 million years, all the way back to when plants made their way onto land.35 Some theories suggest that mycorrhizal fungi supplied all of the nutrients a plant needed as it adjusted to life on land. Roots were originally developed to anchor the plant and only later did plant roots learn to absorb nutrients on their own. Whatever the case, there are two types of mycorrhizal fungi: endomycorrhizal (ones that infect the interior of the root i.e. the famous arbuscular mycorrhizal fungi (AMF)) and ectomycorrhizal (ones that form a net around the outside of the root). Both types greatly increase the ability of crops and pasture plants to absorb nutrients from the soil. In fact, plant roots associated with mycorrhizal fungi increase their access to nutrients in the soil many times over. Their long, spindly bodies, called hyphae, are about 1/60th of the diameter of plant roots, so they are able to explore farther out into the soil and into much smaller crevices.36 Fungal hyphae are also hollow with liquid flowing through the center, which allows for smooth transport of material, resembling water through a pipe. Ectomycorrhizal fungi are capable of producing organic compounds that chisel away nutrients from soil minerals and organic matter, while AMF are not. Ectomycorrhizal fungi are also particularly good at transferring nitrogen, phosphorus and water to plant roots in exchange for carbon and energy. AMF team up with other microbes that solubilize minerals so they can absorb and deliver them to plant roots. AMF are particularly good at transferring phosphorus, copper, and zinc to plants in exchange for carbon and energy.37 This is good news for human nutrition, not just plant nutrition. AMF applications have been found to increase health-giving contents of lycopene, vitamin C, vitamin A and antioxidant activities than non-AMF tomato plants.38
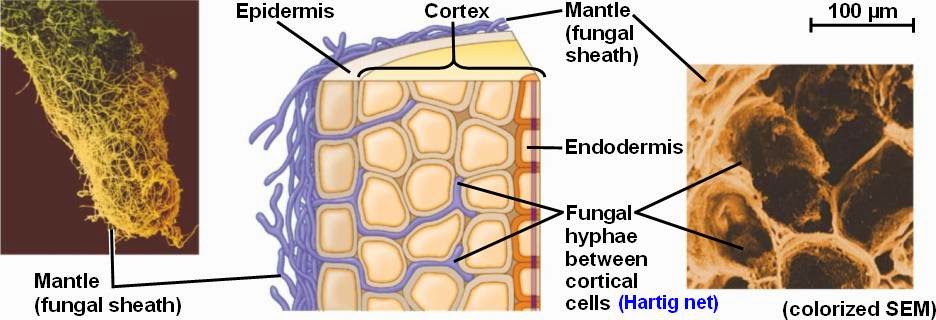

Determining exactly how many nutrients are delivered to plants via bacterial and fungal relationships is a difficult. One scientific paper claims that up to 80% of plant N and P is provided by mycorrhizal fungi.39 Another writes that myorrhizal fungi and solubilizing bacteria are responsible for around 5–20% (grassland and savannah) to 80% (temperate and boreal forests) of all nitrogen, and up to 75% of phosphorus, that is acquired by plants annually.40 Plant uptake of nutrients from symbiotic relationships is a relatively new area of research, so following hard-and-fast guidelines on how many nutrients are made available is not advisable. A good strategy is to take a Total Nutrient Digestion test to understand the total amount of nutrients in the soil profile. Next, take a PLFA test to see the type and amount of microbes in the soil profile. Finally, a Haney soil test will show nutrient levels in their various forms, as well as biological activity, which gives a general idea of how many nutrients will be made available throughout the growing season. Remember, these soil tests aren’t gospel, they’re just snapshots to give a general trendline over time. The best way to know how nutrients are cycling in your context is to run trials with various fertilizer rates and observe the results. Control plots with no fertilizer application provide excellent data on how well the natural nutrient cycle is functioning. It’s advised that producers run trials on the amount of crops or pasture that will allow them to sleep at night! The goal is to decrease fertilizer rates slowly and responsibly over the years. The ability for soil biology to cycle nutrients wasn’t diminished overnight, and it’s recovery won’t happen overnight either.
So, let’s imagine a cover crop is actively pumping food into the soil system and bacteria and fungi are feeding and proliferating like mad. This is a desired outcome, but, as with all things in life, there’s such a thing as too much of a good thing. Bacteria and fungi populations will eventually run amok and hurt plant productivity due to the nutrients immmobilized in their little bodies. Think about the benefits of wild ruminants and the damage that can be done when deer and other ruminant populations are high. Thankfully, anytime there is a food source in nature, there is something right around the corner that eats it. With wild ruminants, there are wolves, coyotes, bears and wild cats to keep their populations in check. In the soil, protozoa and nematodes are the primary predators that keep bacteria and fungi populations in check. Protozoa are part of the “protists”, one of the six kingdoms of life. This means they are separate from bacteria and fungi categorically. Nematodes are worms, just like earthworms, so they are in the animal kingdom. Before you go out and buy a nematocide to get rid of them, it’s important to realize that nematodes help us way more than they might hurt us. You wouldn’t make a dent in their population anyway. Nematodes are the most abundant animal species on the planet by far, as they make up four-fifths (80%!) of the animals on this planet. For every human, there are 57 million nematodes on the planet.41 If they were bad guys, we wouldn’t be here. Just like bacteria and fungi, we only care about them when a group of nematodes take advantage of an unbalanced system with an abundance of food they can utilize, such as the dreaded soybean cyst nematode.
The truth is that protozoa and nematodes are overwhelmingly helpful to the natural cycling of nutrients due to their consumption of bacteria and fungi. In the same way that manure from livestock benefits plant nutrition, protozoa and nematodes excrete nutrient-rich manure that plants can utilize. The most well-studied nutrient that plants receive from this process is nitrogen. All living organisms arrange their cells such that there is a constant amount of carbon and nitrogen. This is called the Carbon-to-Nitrogen ratio (C:N, for short). Bacteria have a very low C:N ratio, meaning their cells are chock full of nitrogen compared to nitrogen. Some call bacteria “little bags of fertilizer” for this reason. Fungi have less nitrogen in their cells, but they still have more than their predators. Therefore, a protozoa or nematode will consume more nitrogen than it needs, which gets released in its waste in the form of ammonium (NH4+), a very plant-available form of nitrogen.42 Protozoa and nematodes are also important because they release nutrients from soil organic matter and have been found to consume each other! Research even shows that nematode presence affects root growth, as increased populations induced more branching and thinner roots in tomato seedlings compared to control soil nematode populations.43 Thinness is a desirable trait for roots because it allows for a better interface to transfer nutrients in and out of the root. In a similar vein (ba-dum-tss!), this is why human capillaries are one cell thick. Oxygen in the lungs can diffuse through the cell and enter the bloodstream to attach to red blood cells, which can deliver oxygen to the rest of the body. Nutrients in the soil can enter thin roots easily, while other nutrients move outward from the roots to the soil. In addition, numerous and thin roots vastly increases the surface area that can come into contact with soil nutrients.
One final point to touch on is the need for good soil structure. Protozoa and nematodes are much larger than their prey, so they require good soil aggregation to create macropore space big enough for them to swim through. They are also subaquatic creatures, so they require good aggregation to hold water in a healthy manner. This allows them to move through the thin films of water and find their next meal. Compacted soil structure hinders the ability of these predators to move around and swim, which slows down the cycling of nutrients. Yet another of the thousand reasons why soil aggregation is beneficial for producers. For more information on protozoa and nematodes and why they rock, check out this informative article from The Ohio State University.

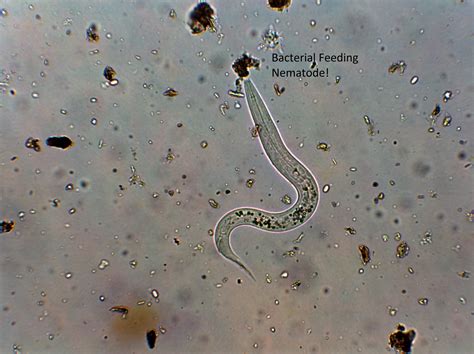
Rising nematode and protozoa populations attract bigger organisms that consume them. These larger predators ensure that the Nutrient Cycle keeps moving along nicely. Springtails and mites are common predators at this stage in the Soil Food Web. In addition to their role as predators, these microarthropods also decompose organic matter and shred plant residue into smaller pieces. Shredding speeds up the release of nutrients from plant residue by increasing the amount of surface area available for microbes to access and decompose. This is why shredding leaves on a lawn causes them to break down much quicker, and one reason why tilling in crop residue results in much quicker decomposition.
Earthworms are another critically important animal in the Nutrient Cycle. These annelids mainly consume plant litter, microbes and soil. In fact, earthworms consume 2-30 times their weight in soil every day. The digestive system of earthworms is designed to squeeze, shred, enzymatically decompose and compress bits of food together as it passes through them. Bacteria and fungi that inhabit the digestive system also aid this process. As a result, earthworm manure, called castings, is a hotspot of organic matter, plant- available nutrients and biological activity. Some earthworms leave castings on the soil surface, while burrowing earthworms line their tunnels with them. Plant roots easily grow through these tunnels and receive a treasure trove of nutrients for their efforts. Burrowing earthworms also reduce nutrient loss, particularly nitrogen loss, by dragging animal manure and plant residue down into the soil profile where it is safe from erosion and volatilization. Lastly, earthworm bodies themselves contain large quantities of nutrients which are made plant-available upon their death and decomposition. Research shows that plants growing in soil with large earthworm populations may take up 45-80 lb/acre (50-90 kg/ha) of nitrogen through this process.44,45 Take advantage of these keystone creatures if they are common in your area.
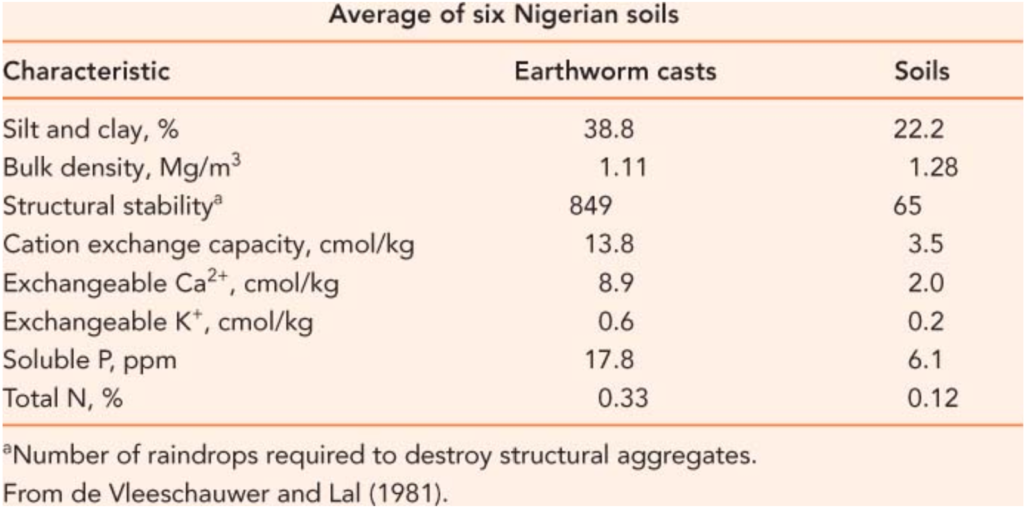

Rhizophagy
In an unforseen turn of events, research now shows that a new member of the soil community consumes bacteria and fungi for nutrition. The other ferocious predators? Plants. (Sorry vegans and vegetarians…) Dr. James White of Rutgers University and his team published their game-changing research on what’s called the “Rhizophagy cycle (“Rhizo” meaning root; “phagy” meaning eat)” in 2018. Here’s how the rhizophagy cycle works. Plants attract bacteria and yeast-like fungi around the tip of growing roots by exuding organic compounds. These organisms enter the root and begin producing a hormone called ethylene. This ethylene causes root cells to produce a compound called superoxide (H202), a reactive oxygen species (ROS). ROS are dangerous for cells, which is why anti-oxidants are good for us! In this case, superoxide is helpful for the plant because it reacts with microbes by stripping off their cell walls. Enzymes in the root go to work by dissolving these cell walls into individual nutrients. Cell walls contain appreciable levels of carbon, hydrogen, oxygen, nitrogen, iron, zinc, phosphorus, potassium, sulfur and many other nutrients! In addition, cell wall-less microbes produce nitric oxide (NO), which eventually becomes nitrate (NO3-) that plant cells can absorb as an additional source of nitrogen.
Some naked microbes die in the root, while others survive and multiply. These microbes continue to produce ethylene, and when enough group together, the high concentration of ethylene triggers the outermost root cells to expand. Sound familiar? These are root hairs! Root hairs are not special cells that are separate from the rest of the root. They are just long extensions of the outermost root cell, called an epidermal cell. Microbes become trapped in the root hair as it expands outward until eventually they are ejected out of the tip of the root hair, along with superoxide and ethylene. Root hairs will eject microbes several times during its life cycle. Once ejected, microbes retrieve nutrients in the soil and start the process of rebuilding their cell walls. These microbes could very well find themselves in the root once again, where plants can strip and absorb their hard-earned nutrients, thus completing the rhizophagy cycle. Fascinating, isn’t it? Very few people 20 years ago would have imagined that plants farm microbes for their nutrients or that root hair production might be due to microbial hormones, making the absorption of water and nutrients by root hairs a happy byproduct, not its raison d’etre.
The million dollar question that producers need answered is whether or not the rhizophagy cycle supplies a good amount of nutrients to plants. If it doesn’t supply a significant amount, then it’s a fun story that doesn’t help much (from a production standpoint). It’s still early days, but initial research by Dr. White and his lab suggest that grass plants acquire 30% of their nitrogen from the rhizophagy cycle.46 His research also suggests that absorption of metal elements like zinc, copper, iron and magnesium from microbes is an important benefit of the rhizophagy cycle. Stay tuned, because future results might surpass our wildest expectations.
For further information on the rhizophagy cycle, check out this great talk by John Kempf at the National No-Till Conference. John explains how he regularly sees producers cut their nitrogen bill by 30-70%, and in some cases 100%, by maximizing biological nutrient cycling. Of course, this is anecdotal, but there’s no reason it couldn’t be true given everything we’ve learned so far. Teaming with Bacteria by Jeff Lowenfels is also an excellent book that dives deep into the details of rhizophagy.
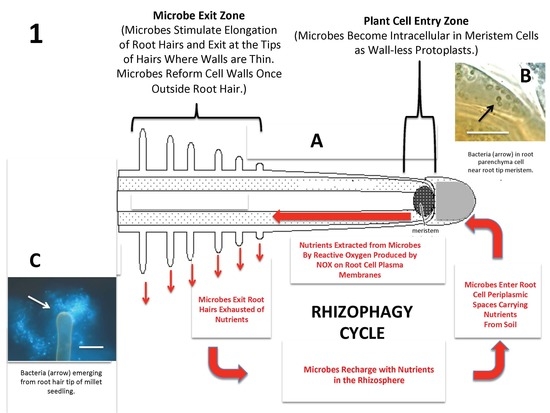
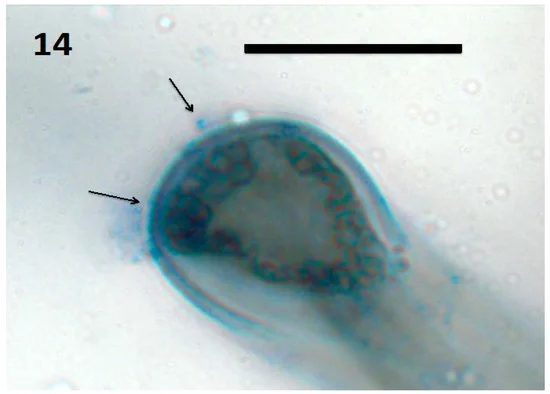
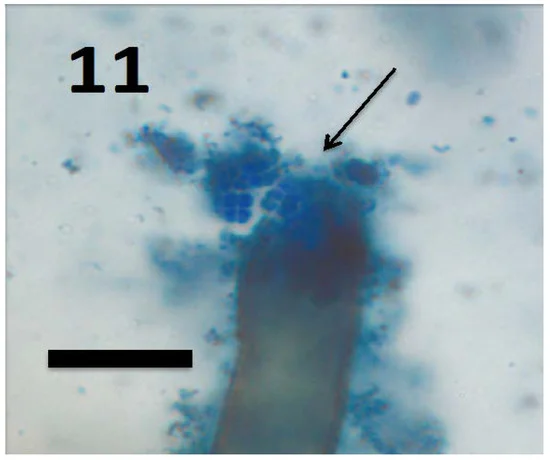
The Importance of Organic Matter
If there’s a singular takeaway message from this article, it’s that the Nutrient Cycle needs living organisms to function properly. Roots, microbes, earthworms and all the rest of the underground herd transform and translocate nutrients at a ferocious pace compared to soil without biological activity. In fact, that’s essentially what staying alive means: the continual transformation and translocation of nutrients in order to gain energy and raw material. Pretty simple, but what happens when soil organisms die? More importantly, what happens to all of those valuable nutrients? To paraphrase the words of my university Ecology professor, “From a nutrition standpoint, the best diet for an organism is one of its own kind. The amount and ratios of nutrients that are unique to each species are already found in their bodies.” While I’m not usually one to argue for cannibalism, there is a point to be made that a dead organism contains carbon, hydrogen, oxygen, nitrogen and all the rest in one convenient location. Compare this to the bulk soil where those nutrients are scattered about and likely in a hard-to-access form. For this reason, many soil organisms exude enzymes that break down dead plant residues, dead microbes or dead animals. The bodies of dead organisms lose their easily decomposable parts first, while the more chemically resistant parts are left intact. Another common outcome for dead and decaying biology is to adhere magnetically to the exterior of a soil particle or get trapped inside of a soil aggregate. Both locations physically protect them from biological breakdown. Chemical breakdown and physical protection leaves the soil looking like a graveyard strewn with dead bodies in various stages of decomposition. As spooky as that sounds, this is an excellent way to increase nutrient cycling efficiency in the soil, because organic matter is primarily composed of dead and decaying material from organisms.47
Organic matter is one of the most important factors in a soil’s ability to hold and distribute nutrients, which is quite astounding considering it comprises less than 5% of soil in most places. One reason why organic matter punches above its weight is because of its high Cation Exchange Capacity (CEC). Recall that chemistry is simply the movement of electrons. Certain compounds and atoms readily give or take electrons to become more stable. The gain of electrons brings more negativity because electrons carry negative charge. Think about receiving debt. You “gain” something, but it is a negative to your bank account. Atoms that gain extra electrons and become negatively charged are called “anions“. On the other hand, losing electrons results in a positive charge. Atoms that have a positive charge are called “cations“. CEC is a numerical value representing the ability of a soil to hold on to the various cations in the soil, such as Calcium (Ca2+), Magnesium (Mg2+), Potassium (K+), Sodium (Na+), Aluminum (Al3+) ammonium (NH4+) and others. Organic matter has a much higher CEC than sand, silt and clay, which means there are many more negatively charged sites that magnetically attract positively charged nutrients. Thankfully, organic matter took .38 Special’s advice and holds on loosely to these nutrients. This allows roots and living organisms to easily remove these nutrients and consume them when they need to. The reason that organic matter has such a high CEC in the first place is that the decomposition of organic matter leaves compounds in smaller, fragmented bits, meaning there is a lot more surface area and a lot more exposed, charged molecules that can attract polar (positively or negatively charged) molecules. Some compounds, such as fats and lignin, have no charge until they are partially decomposed, so they don’t attract charged nutrients when they are intact, like how wood doesn’t attract magnets. This is also why organic matter dramatically increases a soil’s ability to hold water (water has positively charged and negatively charged regions). Finally, Anion Exchange Capacity (AEC) also increases. AEC is not talked about as much as CEC, but it is a critical function in the soil. The soil has a natural negative charge that repels negatively charged nutrients, such as nitrate (NO3–), sulfate (SO42-), phosphate (PO43-) and boron (B(OH)4−), which means these nutrients are constantly at risk of leaching out of the soil if they are not taken up by living organisms or magnetically connected to positively charged sites on organic matter. Therefore, organic matter saves producers money by ensuring valuable nitrogen, sulfur, phosphorus and boron don’t end up in the nearest river.
So, organic matter is chock full of nutrients because of its electrical charges and because its individual components were once living biology or compounds produced by living biology. To a soil inhabitant, this makes organic matter the ultimate buffet (more like the ultimate bed and breakfast if you consider organic matter’s ability to promote good soil structure at the same time). Nutrients from organic matter become available to living organisms over time as microbes and other soil organisms nibble away at exposed surfaces. Assuming adequate biological activity, organic matter should be viewed as a slow-release fertilizer that can provide significant quantities of nutrients to plants over the growing season. According to the values in the image below, a soil with 5% organic matter and good biological activity is expected to supply around 100-150 lb of nitrogen per acre per year. This is enough nitrogen to fulfill the nitrogen needs of the grain from 200 bushel of corn, which requires 134 lb of nitrogen per acre at that yield level.48 20-35 lb of phosphorus and 10-15 lb of sulfur would also be supplied for free by biology, as long as it is active.
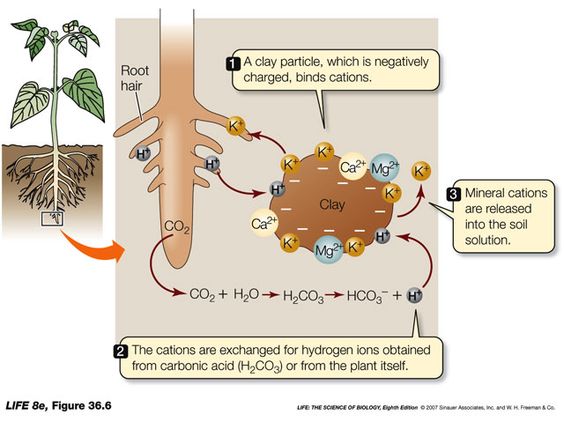

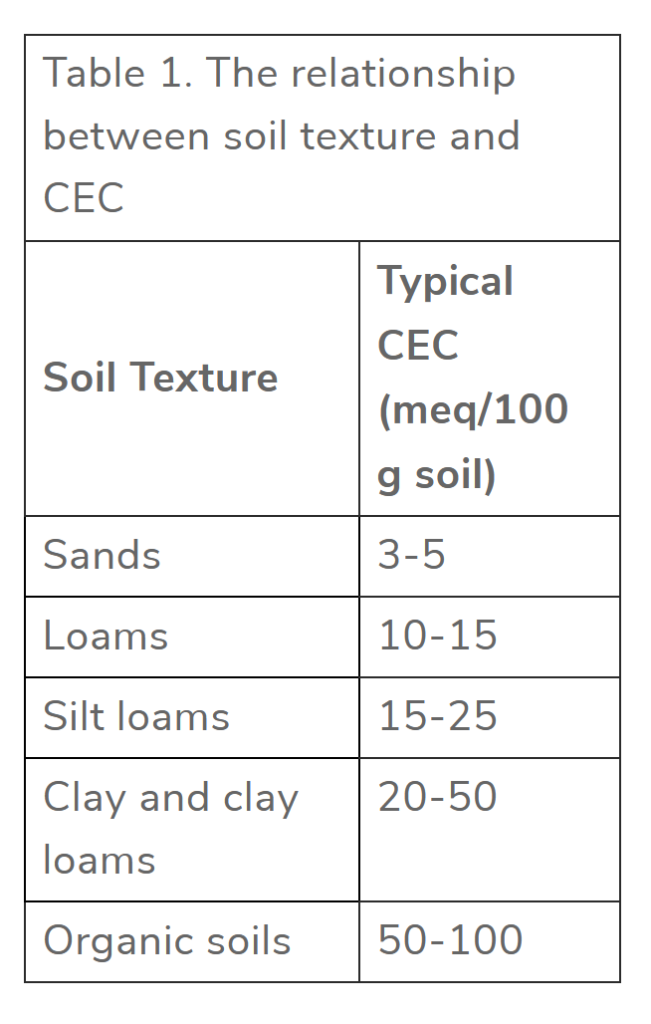
Unfortunately, soil organic matter levels have slowly decreased over the past 200 years in agricultural soils.49 One practice particularly good at catalyzing the depletion of organic matter levels over time is tillage. There are two main reasons why this is the case. First, the practice of tillage infuses oxygen into the soil. Aerobic (oxygen-dependent) microbes are the most efficient decomposers of organic matter, so adding oxygen to the soil is like adding fuel to an already burning fire. Second, the physical obliteration of soil aggregates exposes once-hidden organic matter to the hungry masses. These two factors lead to a feeding frenzy of organic matter for its energy and nutrients. Most of the carbon inside organic matter leaves the soil as CO2 gas when microbes consume it, while other nutrients immobilize and mineralize unnaturally fast as well. Crops in this situation are able to absorb these nutrients fairly well, which is why crops in tilled virgin ground grow better than surrounding areas for a few years. However, all good things must come to an end. Fields and pastures become less and less productive as carbon continues to float away and other nutrients leave the field via the crop, animal, air or water faster than they are replaced. External nutrients (a.k.a. fertilizers) become necessary at this point if producers wish to sustain yields. Whether or not these fertilizers decrease organic matter over time is still being debated. Some research shows that increased plant production more than offsets the negative effects of fertilizers50, while others claim they do decrease organic matter levels.51
Carbon to Nitrogen Ratio
Much of the first half of this article was spent attempting to undo decades of nitrogen bias, so allow me to put this section in its proper context and not seem like I’m speaking out of both sides of my mouth. Nitrogen is hugely important because it’s a building block in the DNA, RNA and proteins of all living organisms. Therefore, nitrogen is needed in larger amounts compared to nutrients not named carbon, hydrogen and oxygen. The point made earlier was that the rest of the nutrients have different jobs, and they are all required if an organism is to be healthy and well. Carbon, hydrogen, oxygen and nitrogen make up the bulk of biological compounds, like plastic and rubber used in a TV remote control. Other nutrients, particularly the metals, are needed in much smaller quantities. These nutrients facilitate electron movement (a.k.a. chemical reactions), which allows biological compounds to function, like the zinc, potassium and manganese atoms inside of the AA batteries that cause the TV remote control to do its job.
With all of that said, carbon and nitrogen’s quantitative prowess make them uniquely important as biological compounds decompose. Carbon is the backbone of all major biological compounds, and living organisms need a constant intake of carbon for two reasons. First, they need it as a raw material to build their own biological compounds. Second, living organisms gain energy to do things by breaking apart carbon bonds and harvesting the energy. This is why many people equate carbon with energy. Check out this article on cellular respiration to see how aerobic organisms like us accomplish this task. Nitrogen, on the other hand, is mostly important for its function as a building block. Therefore, a handy way to think about energy and building material is to calculate how much carbon and nitrogen are contained inside of an organism. We call this the Carbon to Nitrogen (C:N) ratio. This concept was briefly introduced when discussing bacterial C:N ratios versus predatory nematode C:N ratios. Plants also differ in their C:N ratios. Young, green plants are made up of nitrogen in a decently high ratio. As plants age, carbon accumulates in the form of lignin and cellulose, which are long chains of hard-to-decompose carbon structures. These carbon-based molecules allow plants to stand up as they grow bigger and heavier. In essence, they behave like the plant’s skeletal system. Young plants don’t have as many of these compounds quite yet, so they are mainly composed of simple, easy-to-break down compounds higher in nitrogen. This is why they have low C:N ratios.
If you are confused, think about a 1:1 ratio (1 Carbon for every 1 Nitrogen). Now, let’s add 9 carbons and 0 nitrogens to our 1:1 ratio, for example. This gives a 10:1 ratio (10 carbons for every 1 nitrogen). Take a look at the chart below to see actual values.
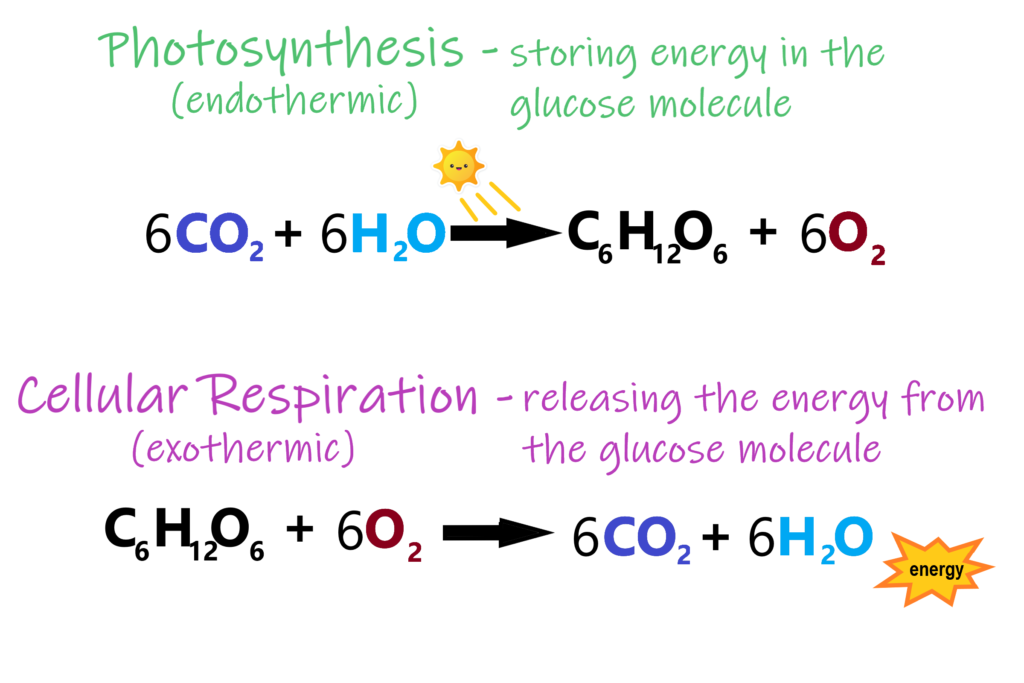
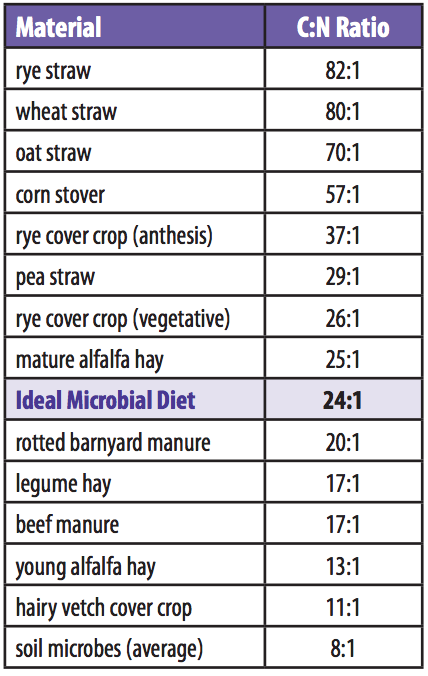
Farmers and ranchers already have an idea of the buildup of complex carbon compounds in mature plants because wheat straw takes much longer to decompose compared to young, green plants. Farmers and ranchers also know that their crops often show signs of nitrogen deficiency when they are planted into wheat, corn or rye stubble. The reason is that decomposing bacteria and fungi have C:N ratios around the 3:1-10:1 range. This means the amount of nitrogen in their bodies is fairly high compared to the amount of carbon. Compare this to wheat straw which has a C:N ratio of 80:1, meaning there are comparatively fewer nitrogen atoms compared to the amount of carbon. Bacteria and fungi that consume wheat straw accumulate carbon much faster than nitrogen. This carbon is used in energy production and as building material in the construction of carbohydrates, proteins, genetic material and lipids. So, imagine the construction of proteins and genetic material begins. Carbon locks into place as the backbone of these molecules, but there is not enough nitrogen to finish construction. Bacteria and fungi are then forced to scavenge the soil for accessible nitrogen to finish the job. Due to their large population sizes, these microbes consume accessible nitrogen quickly, which leaves very little for plant roots to absorb, and we see symptoms of plant nitrogen deficiency.
On the flip side, microbes are forced to scavenge the soil for carbon when decomposing organic material with low C:N ratios. This is the case for high protein crops, like our legumes. So, imagine a field of young alfalfa is terminated and decomposing. Microbes consume a plethora of nitrogen, which they utilize to build protein and genetic material. However, the young alfalfa does not provide enough carbon to complete the construction of these molecules, so microbes are forced to scavenge the soil for carbon! This can lead to soil compaction because a common form of accessible carbon is the carbohydates52 and glycoproteins53 (glycoprotein = Carbohydrate + protein molecules. glyco means carbohydrate; Protein means… protein) that hold soil aggregates together. Think of these structures as a mesh bag holding a group of small pebbles together. In this analogy, microbes consume the mesh bags, which allows the pebbles to spill out. The longer microbes aren’t fed carbon, the more mesh bags are consumed and the more pebbles pile on top of each other. Proper aggregation is essential for good soil structure, which provides a healthy mix of aerobic and anaerobic sites for nutrient cycling. Nitrogen-fixation, for example, requires anaerobic conditions! Soil compaction and loss of soil aggregation is devastating for larger members, such as protozoa and nematodes that require macroaggregation and water to swim and find food. This potential collapse of soil structure is why producers are cautioned against planting cover crop mixes too high in legumes and brassicas. Just like everything else, it’s all about balance.
Turn your attention to the equation for cellular respiration above. This is the process by which aerobic organisms use oxygen to unlock energy from carbon-based glucose (a sugar). This process explains why the ideal microbial diet is residue with a 24:1 ratios. Cellular respiration results in the loss of around two-thirds of the total carbon consumed. These carbon atoms gas off as carbon dioxide (CO2). This leaves only one-third of the carbon to be used as raw building material as decomposers break down residue. So, if we assume the average C:N ratio of a bacteria or fungi is 8:1, they would ideally consume material with a 24:1 C:N ratio because two-thirds of the 24 carbons (16 of them) will be released as CO2, which leaves 8 carbons for every 1 nitrogen. Perfect!
The rate of decomposition determined by C:N also affects the availability of calcium, phosphorus, manganese and a whole host of other nutrients because plant residues are made up of more than just carbon and nitrogen. It’s just that those two nutrients play the biggest role in determining when everything is broken down and released.
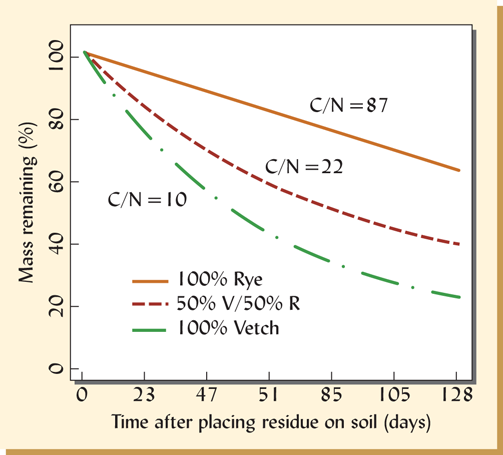
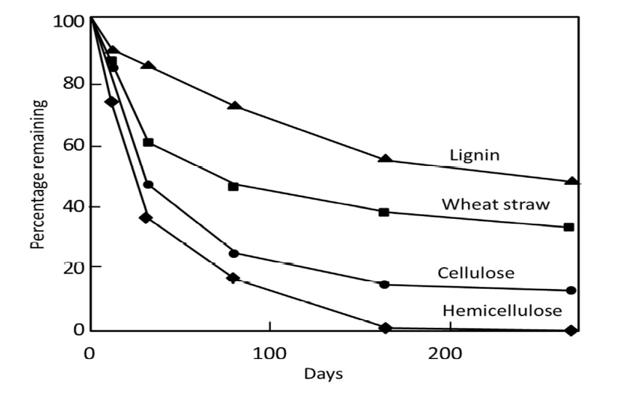
Aboveground Herd
It’s finally time to rise above the hidden world beneath our feet and talk about things we can actually see and touch: our beloved aboveground animals. Just thinking about them puts a smile on a producer’s face. Can you blame them? I mean, who can resist their big, loving eyes, their rugged horns, their intricate mouthparts, their tough, shiny exoskeletons or their six tiny legs? Of course producers love and cherish the billions of insects on their land that work so hard to keep nutrients cycling efficiently, right?! If only…
The truth is that many producers immediately think “pest” when they hear the word insect. They’ve felt the bite of an annoying mosquito, and they’ve felt the financial sting of hungry bugs wiping out their crops. Therefore, they are our sworn enemy, and the appropriate course of action must be to declare war on insects. Putting aside the fact that waging war on nouns is a bad idea from the get-go, trying to fight against these creatures is a losing proposition. Decades of all-out chemical assault and habitat loss has led to significant insect population declines.54,55 Even worse, one study found that “Pests were 10-fold more abundant in insecticide-treated corn fields than on insecticide-free regenerative farms, indicating that farmers who proactively design pest-resilient food systems outperform farmers that react to pests chemically.”56 Scientists are even observing pollinators fleeing the countryside for refuge in the safehavens of cities.57 In the words of Joel Salatin, “This Ain’t Normal, Folks“.
Vilifying insects ultimately means we lose access to the nutrient cycling services they could have provided to us. Consider the words of 20th century scientist William Albrecht to understand the incredible service they provide. He said, “Insects are Nature’s garbage collectors, and diseases are her cleanup crew.” In other words, insects (and disease-causing microbes) are opportunists that only consume food they can digest. The digestive systems of insects are not very complex, and they are only able to digest simple molecules in most cases. Think about fruit flies and bananas sitting on the counter. We only see evidence of fruit flies once the bananas begin to rot. This is because healthy bananas contain complex molecules that fruit fly enzymes cannot break down. These molecules break apart into simpler compounds during the rotting process, which the digestive systems of fruit flies can handle. Nature’s garbage collectors! Their job is to consume the nutrients found within the rotting bananas and recycle them back into the realm of the living. In the wild, insects (and the microbes they harbor in their guts) are key players in the decomposition of dead plant and animal material.
The hard truth is that the same process occurs in unhealthy crops and forage plants. Insects are simply identifying and consuming sources of nutrients their digestive systems can handle. John Kempf’s “Plant Health Pyramid“™ does a good job of describing this process. In a nutshell, healthy plants are able to produce complex carbohydrates, complete proteins, more lipids and more secondary metabolites which insects cannot handle. Proper nutrition is an essential component for a plant to produce these compounds.
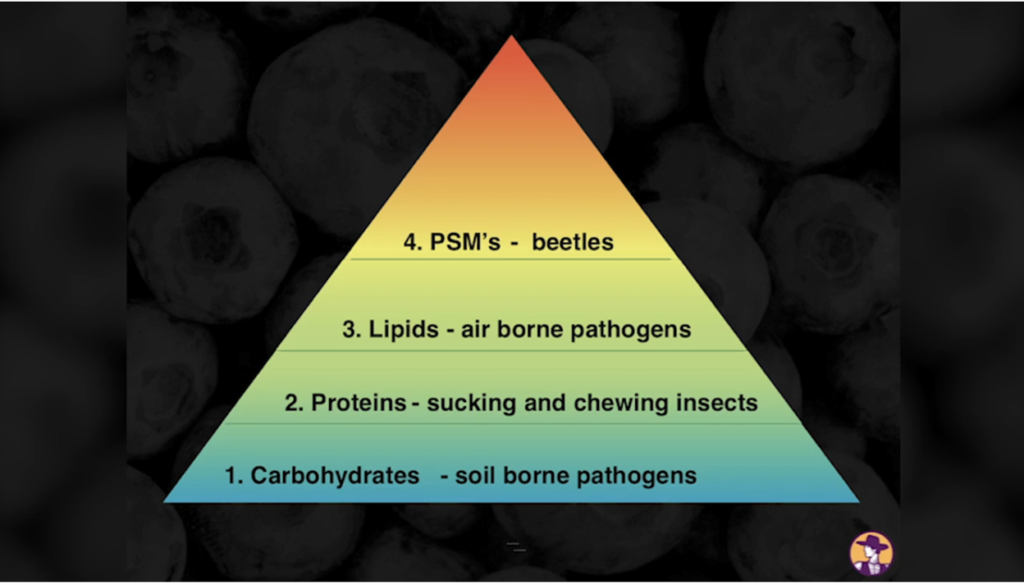
It’s high time we sign a peace treaty with insects and give them the freedom to play their God-given role in the Nutrient Cycle. Less than 1% of all insect species are pests58, and many of the other 99% consume them as a food source. Not only does increasing habitat and reducing insecticide usage keep the pests in check, the Nutrient Cycle is balanced and sped up over time. In fact, research shows a healthy level of insect pressure can have a pruning effect and increase plant production through better nutrient cycling.59 Nature’s little garbage collectors are just making sure that nutrients get taken out of unhealthy plants so they can be recycled quickly into the next generation. For that, we should say, “thank you, insects. Nutrients are cycling much more efficiently with you in the world.”
Another important service that insects provide is their ability to shred organic material into smaller and smaller bits. This increases the surface area that microbes have access to and, consequently, increases the speed of mineralization of nutrients.60 Insects not only shred organic material, but they drag much of it into the raging inferno of microbial activity we call the soil. The most famous example of this phenomena is the dung beetle. Dung contains high levels of organic matter and nutrients, and the fate of these materials greatly depends on the action of insects. For example, much of the nitrogen inside a dung patty will volatilize (turn into a gas) and leave the pile if left untouched. Volatilized nitrogen cannot be used for plant growth. Aboveground, dung beetle activity has been shown to decrease volatilization and ensure most of the nitrogen is recycled back into living organisms61 by aerating the dung pats, which alters the population of microbial decomposers.62 Belowground, microbes feast on the organic matter and nutrients drug into the soil. This increases the pool of available nutrients in the soil, including phosphorus and potassium, whose content in soils is strongly influenced by dung beetle activity.63 Termites have a similar positive effect on the Nutrient Cycle, especially with respect to nitrogen. 64
Nutrients that insects consume become available again once their bodies and excreted molecules are decomposed by microbes, or when they are consumed by other animals, such as spiders, birds, moles and other insects. Ladybugs, green lacewings, praying mantises and ground beetles are common predators that feast on other insects.

From single-celled microbes to large animals, the Nutrient Cycle is a beautifully intricate tapestry of eating and excreting. Speaking of excreting, the management of livestock manure is one of the most important factors dictating the efficiency of the Nutrient Cycle in a crop field or pasture. These patties and pellets are teeming with nutrients and biology! On average, 70-80% of the nitrogen (N), 60-85% of the phosphorus (P), and 80-90% of the potassium (K) in animal feeds are excreted in the manure.65 Whether or not these nutrients support future plant growth depends upon how it’s been managed. For example, surface applied manure loses 100% of its ammonium (NH4+) to volatilization, while incorporated manure loses ammonium much slower.65 Another factor to consider is whether the manure was allowed to fester in a lagoon prior to application. Up to 80% of the phosphorus in a lagoon sinks to the bottom, so fertilizer spray made from the liquid is a much lower source of phosphorus.66 Lastly, producers should always understand which species of livestock their manure came from because livestock classes excrete varying concentrations of nutrients. Check out the graphic below from Oklahoma State University to get an idea of how manure type and species of origin affect nutrient levels. Obviously, NPK are not the only nutrients contained within manure, but they are the most heavily studied. Beneficial metal nutrients like sulfur, copper and zinc have also been found in manure. Unfortunately, toxic levels of heavy metals may also be lurking in the muck, so producers should try their best to know the nutrient content of manure before it is applied.67,68 In a nutshell, manure is not manure is not manure.
The digestion of plant material by livestock can be thought of as a super-charged composting process that dramatically speeds up the cycling of nutrients. Livestock digestion is especially important for cycling nutrients in drier landscapes, because low moisture content slows down the natural decomposition of plant material. Without animals, plants and the nutrients contained inside of them cycle too slowly to support vibrant growth. Arid landscapes need consumers to speed up the cycling of nutrients and provide manure piles that contain the moisture, biological activity and nutrients that a new generation of germinating seed requires to get off to a strong start. Allan Savory and the team at Holistic Management have seen this play out on their experimental plots in Zimbabwe. They maintain a high stock density of animals on a barren piece of ground for seven nights and then take the animals off. The results of this practice are the build-up of soil, better cycling of nutrients and a flurry of new plant growth over time. They call this a “kraal“, and many of the benefits, such as an 8-fold increase in carrying capacity of the land, are attributed to the nutrients from the manure and urine laid down. Rancher Alejandro Carrillo has documented a similar increase in nutrient cycling from animal impact on his ranch in the Mexican desert, as you can see from the images below.



The great thing about livestock is that manure is only one mechanism through which they can improve the Nutrient Cycle. The physical trampling of plant material onto the soil surface is another indispensable service they provide. Decomposing bacteria and fungi need plant material to be physically contacting the soil surface if they are to unlock and recycle their energy and nutrients. Check out this video from the woods showing the difference in decomposition between the underside of woody material and woody material that is not touching the soil. The same process happens to crop residue and forage plants. Standing plants return their nutrients to the soil exponentially slower than plants physically touching the ground. Even worse, nutrients in standing plants eventually gas off, thus reducing the amount of total nutrients in the system. These “oxidized” plants are also a hindrance because they’re taking up space where growing plants could be photosynthesizing and providing energy to the soil that could be used to cycle more nutrients. Lose-lose-lose.
Livestock also contribute to the Nutrient Cycle by adding copious amounts of beneficial microbes onto plant residue and onto the soil. These microbes are added via manure, urine, saliva and general shedding from their bodies and feet.69 When you stop and think about it, the benefits that livestock can provide to the Nutrient Cycle are astounding. Though, it makes perfect sense because animals, plants, microbes and abiotic forces developed together for eons. They all provide crucial services and rely on each other’s services for food and energy. Thankfully, systems-based research is mounting that corroborates the claim that properly managed (one might say regeneratively managed) livestock are positive for the Nutrient Cycle of an ecosystem. Each successive study that comes out is like another piece of the puzzle, revealing a more complete image. Results from the past few years of research show that livestock can catalyze soil microbial biomass70, soil nutrient availability71, soil carbon and nitrogen cycling72, insect diversity and species richness73, plant species diversity74 and bird abundance75. Research also indicates that regeneratively managed livestock reduced the average annual surface runoff, sediment, Total Nitrogen and Total Phosphorus loads in a Texas watershed outlet by 39%, 34%, 33% and 31%, respectively.76 These are all positive signs that nutrients are kept in the system and the pool of available nutrients in the soil is increasing, which allows a landscape to support a higher population and diversity of life. Win-win-win.
Yeah, but...
Yeah, but won’t we mine the nutrients out of our soils? There are two basic schools of thought when it comes to plant nutrition. Mainstream agricultural knowledge says that plants can only absorb inorganic, water-soluble nutrients. While plants do absorb inorganic, water-soluble nutrients, only a fraction of nutrients in the soil are found in these forms. Combine that with the fact that traditional soil tests only measure this tiny pool of nutrients and you can see why there is a genuine concern about mining them out. On the other hand, we now have a better understanding that plants also absorb nutrients in organic forms and that they regularly do so. The other school of thought contains those that believe the majority of nutrients are absorbed in organic forms, such as from intact amino acids and nutrients in the cell walls absorbed through the Rhizophagy cyle. In addition, newer soil tests, specifically the Total Nutrient Digestion (TND) test, allow producers to see the full reservoir of nutrients that exist in their soils. Tapping into these pools is determined largely by the biological activity of the soil, which producers can learn more about from taking a Haney soil test and a PLFA soil test. So, to answer the question, it depends who you ask, really. Plant nutrition is extremely complex, and there are numerous variables at play, so it’s likely that the truth lies somewhere in the middle. Stay tuned for more research. In the meantime, on-farm experimenting and annual soil testing gives producers powerful data showing trends over time based on various practices and fertilizer application rates.
Yeah, but how many of those unavailable nutrients can actually be provided by natural nutrient cycling? This is another great question and unfortunately it’s tough to say at this moment because the field of soil microbiology is in its relative infancy. Take viruses, for example. Research indicates that viruses kill 20% of ocean bacteria every day(!!), which significantly impacts aquatic nutrient and energy cycling.77 As important as they are, very little is known about the impact that soil viruses have on the terranean and subterranean Nutrient Cycles. Early results are positive, as they show soil viruses play a beneficial role in releasing immobilized nutrients inside of bacterial cells.78 Once again, stay tuned for research that shows how these invisible processes impact our everyday decision-making processes! Even though we live in relative ignorance to all of the processes happening around us, our knowledge of soil microbes and nutrient cycling has increased dramatically thanks to advances in technology, like DNA sequencing. Fortunately, we don’t need to know every little detail before we can improve nutrient efficiency on the farm, ranch, golf course, lawn or garden. Regenerative producers like Gabe Brown and Rick Clark are showing that practices like planting cover crops and reducing tillage can lead to a dramatic decrease and, in their cases, an elimination in applied fertilizer. Pioneering producers like them have put their time and money on the line to discover which practices work. Research is now trying to catch up with the “how” and “why” of it all.
Yeah, but why shouldn’t I just follow my normal soil tests and maintain business as usual? Consider the following quote from the 2021 Illinois Agronomy handbook: “the information generated [from a soil test] typically comes from a sample from the plow layer, but the crop roots extract nutrients below that layer; laboratory precision is typically within 5% to 10% of the true value.”79 Pair that with this quote from the 15th edition of The Nature and Properties of Soil textbook: “relatively little of the fertilizer nutrient (from 10 to 60%) actually winds up in the plant being fertilized during the year of application.” Specifically, more than 80% of applied phosphorus in fertilizer is immediately made unavailable after application.20 At the very least, this level of imprecision and fertilizer use efficiency should spark a desire to do on-farm trials to see where the best ROI is found. There’s a large chance producers are overapplying nutrients. Many farmers begin by running small experiments applying the full recommendation, a half-rate and a control plot. In many cases, the best ROI is in the 30%-50% reduction range in the beginning. These results are of course anecdotal, but it’s still useful information to pass along. We’re all citizen scientists and our “anecdata” is just as important!
Applying excesses of nutrients is also bad business because they can interfere with the uptake of other nutrients and exacerbate nutrient deficiencies in growing plants. Cations (i.e. potassium, copper, zinc, iron, manganese and other positively charged nutrients) are especially antagonistic toward one another as they compete for space in the soil.80 This is a big problem considering conventional fertilizer recommendations have been based off of Liebig’s Law of the Minimum for the past 100+ years. Liebig’s Law of the Minimum states that plant production can be no greater than that level allowed by the growth factor present in the lowest amount relative to the optimum amount for that factor. As a result, “more” became synonymous with “better”. While Liebig’s Law does have a lot of merit, it does not recognize the full scope of nutrient cycling and nutrient interactions in the soil. For example, applied synthetic fertilizer can discourage symbiotic partnerships in the soil. For example, excessive nitrogen and phosphorus fertilizer has been shown to inhibit beneficial mycorrhizal fungi colonization of roots.81,82 Limited colonization means plants can’t reap the myriad benefits that mycorrhizal fungi provide, namely access to water, disease resistance and scavenging for the very nutrients that were applied.
Another reason to ensure nutrients aren’t applied in excess is that overloads of nutrients, especially nitrogen and phosphorus, leave the farm or ranch and end up in public waterways. This leads to eutrophication, which is an overgrowth of aquatic algae that take advantage of the increased nutrient concentration in the water. Over time, these algal blooms consume most of the oxygen in the water and choke out fish, shellfish and marine mammals.83 This has led to various “dead-zones” across the world, including the famous Gulf of Mexico dead zone measuring 3,275 square miles in 2022. That’s more than 2 million acres of habitat potentially unavailable to fish and bottom species — larger than the land area of Rhode Island and Delaware combined.84 Studies show that most of the nitrogen that contributes to the dead zone of the Gulf of Mexico—between 60 and 80 percent—originates on farms and livestock operations in the Midwest, largely in the form of synthetic fertilizers that run off fields of corn and other crops.85,86 Research has also documented a correlation between high levels of nitrates in rural water supplies and negative effects on local human health.87,88
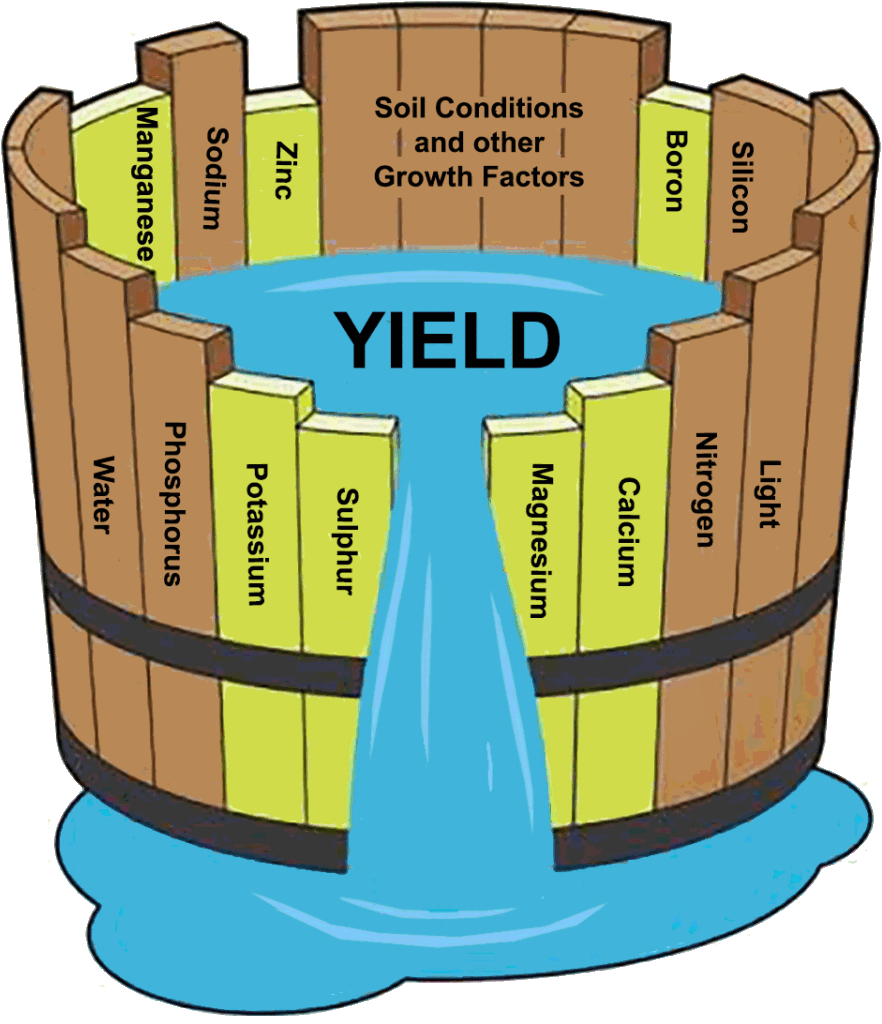
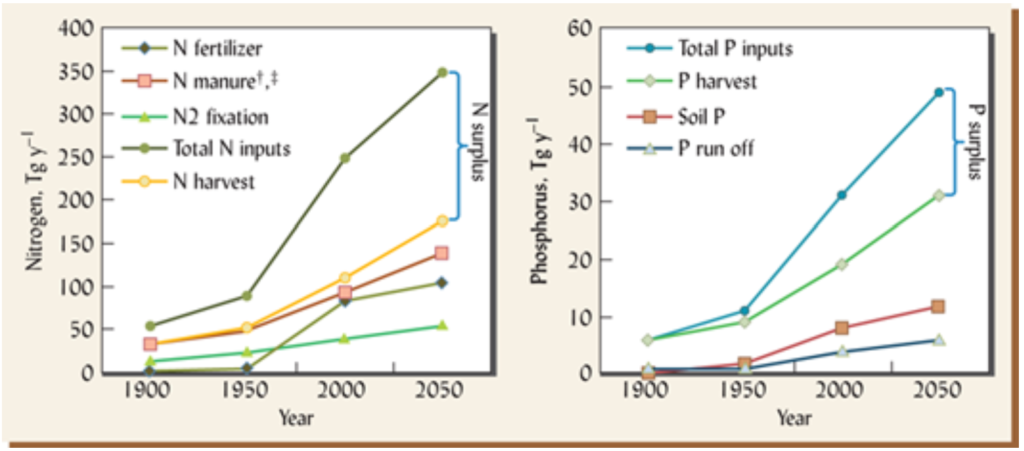
One final note concerning on-farm trials: The Nutrient Cycle in most agricultural soils is not functioning efficiently. This means it’s not wise to quit applying fertilizer cold-turkey. Producers will most likely have a wreck on their hands if they do this. However, it is advised to never skip out on experimental control plots, meaning areas with zero fertilizer application. A ton of useful information can also be gleaned from the control plot. Producers might be surprised at how well the plants perform when given the chance to find their own nutrients. This indicates a healthy soil microbiome. In most other cases, plants suffer tremendously, which means life in the soil is not active and fertilizers are propping up production. These observations will help guide producers along the path of reducing fertilizer use over time by knowing the baseline for their natural Nutrient Cycle.
One additional visual tool is to observe how much soil clings to dug up roots. High volumes of root hairs, good mucilage production, plant exudates, microbial populations and microbial exudates all cause soil to adhere to the roots, in what is called a “rhizosheath”. The biological activity that causes rhizosheaths also indicates a healthy Nutrient Cycle. In the past, farmers and ranchers were told that clean roots indicated healthy roots. We know now that this is a sign of poor nutrient cycling and diminished nutrient acquisition to plants.


Yeah, but farmers need to feed the world. If we all switch to Regen Ag and apply less fertilizer then more people around the world will starve. The agricultural world absolutely needs to take this concern seriously. Research does show that regenerative practices can result in yield depression89 and slower finishing time in livestock90, especially in the first few years of transition. However, other studies are emerging that challenge these positions. Over 40 years of trials from the Rodale Institute found that cash crops fertilized with legumes and manure produced equivalent yield compared to conventionally fertilized cash crops in good weather. On top of that, the cover crop and manure plots exhibited a 30% increase in yield during years with extreme weather.91 As for the livestock concern, Dr. Jackson of the University of Wisconsin breaks down the figures and details why he believes “the United States has enough land to support current beef production with grazed perennial grasslands” while simultaneously building soil health.92 Whether this is true and whether it can be replicated with other classes of livestock remains to be seen.
What we do know is that the nutritional value of the food we’re producing is not brought up often in the “feeding the world” discussion. How much food we produce is purely based on calories. Even so, the world is crushing it at producing enough calories to meet global demand. Estimates show that humans produce 2,947 kcal/person/day globally and 3,782 kcal/person/day in the U.S.93 (2,000 kcal/person/day is the recommended value). That’s great news, but man doesn’t live on calories alone. It’s time now to also focus on increasing the nutrient-density of the food supply. We all know it intuitively because we can taste and see the stark difference between a store-bought tomato a homegrown one, but research is mounting that shows growing food in healthy, biologically active soil is the solution to increasing nutrient-density of our food supply. A landmark research paper published in 2022 tested nutritional values of nine paired farms across the United States: one conventional and one regenerative for each pairing. Averaged across all nine farm pairings the regenerative farm crops had 34% more vitamin K (10% more to 57% more), 15% more vitamin E (11% less to 70% more), 14% more vitamin B1 (17% less to 2 times more), and 17% more vitamin B2 (17% less to 3 times more). The crops from the regenerative farms also had 15% more total carotenoids (6% less to 48% more), 20% more total phenolics (14% less to more than twice as many), and 22% more total phytosterols (25% less to more than 2 times more). In addition, regeneratively grown crops had 11% more calcium (1% less to 43% more), 16% more phosphorus (10% less to twice as much), and 27% more copper (16% less to twice as much).94 Nine farms is a small sample size, but this is encouraging news! Another study from 2021 found that reducing tillage resulted in greater soil fungal populations which translated into greater concentrations of a powerful antioxidant and anti-inflammatory amino acid produced by fungi called ergothionine in corn, soybeans and oats.95 Lastly, 2017 research from Dr. Stephan van Vliet and his team found that “Livestock allowed to graze diverse, nutrient rich forage accumulate higher levels of health-promoting phytonutrients, like terpenoids, phenols, tocopherols and carotenoids in their meat and milk, as well as a healthy ratio of omega 6:3 fatty acids compared to animals allowed monoculture pastures and confined animals finished on high grain diets.”96 Dr. van Vliet documented similar results in this 2021 paper and this 2023 paper.
These incredible research findings highlight the fact that we are an active part of the Nutrient Cycle. Human nutrition starts in the soil, so all of this talk about nutrient cycling pertains intimately to the realm of public health as well, not just agriculture. What we do to the Nutrient Cycle in the soil, we do to ourselves.
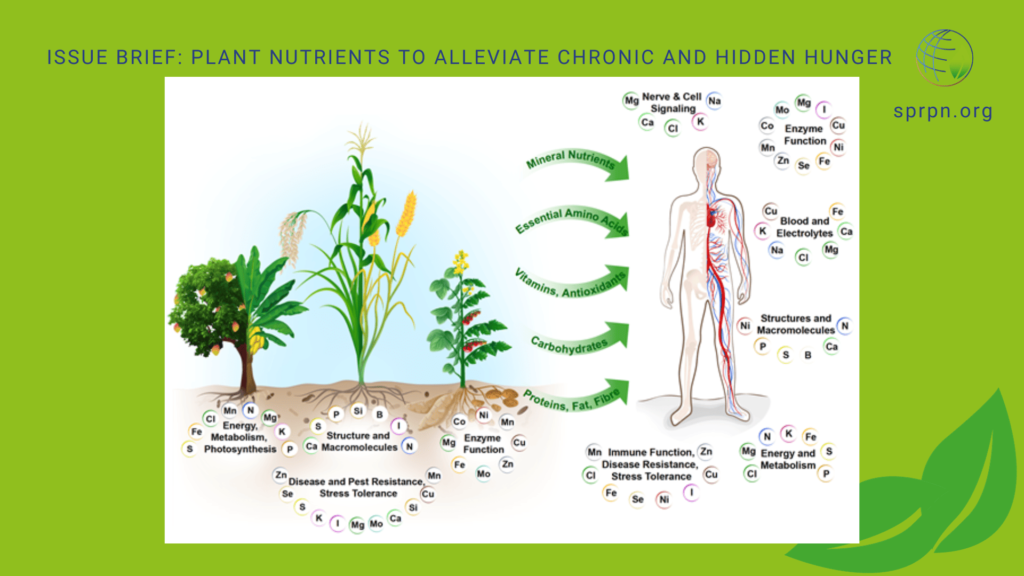
Summary
There’s a famous expression that says, “You can’t manage what you can’t measure”. Managing fertility on a farm or ranch is a prime example. Measurement tools in the 1800’s and 1900’s were not as sophisticated as they are today, so they couldn’t possibly measure what we do now. Today, technology allows us to peer into the complex web of interactions in natural systems that affect agriculture. Even though we’ve made large strides in measuring the invisible and the intricate, there’s a whole lot that we still can’t measure, understand or, consequently, manage. This is humbling for all of us to admit, but we must admit it if we’re being honest. At the very least, discoveries pertaining to the biological importance of the Nutrient Cycle should spark our curiosity and engender a sense of hope that believes we can sustainably decrease the need for outside fertility brought onto the farm or ranch.
It’s also important to remember that fertilizers are products sold by salespeople working for companies that have shareholders who care most about profits on their investments. Fertilizers help feed the world and we shouldn’t abandon them, but it would be naive to think that everyone in the fertilizer industry is in business for purely altruistic reasons. Author Noah Zahn of the Pulitzer Center writes in May of 2023, “Meanwhile, the fertilizer industry has yielded record profits. Canada-based Nutrien Ltd., the world’s leading producer of potash fertilizer, saw profits increase 1575% between 2020 and 2022 to $7.7 billion. Tampa-based The Mosaic Co., one of the largest U.S. producers of potash and phosphate fertilizer, made $3.6 billion in net earnings in 2022, a 438% increase from 2020. CF Industries, an Illinois-based fertilizer company, made $3.2 billion in 2022, a 955% increase from 2020.” Reasons for the surge in profits included COVID transportation chain disruptions, freezing weather in Texas, decreased exports from China, hurricanes, wars and even low water levels in the Mississippi. What this means is that the more producers can’t rely on the natural cycling of nutrients, the more their businesses are tethered to people and events outside of their control. Rather than give away control, regenerative practices build up ecosystem functioning and allow operations to become increasingly self-reliant on the trillions of microbes and animals that will work tirelessly to supply their fertility needs. All they ask for in return is the right type of food and a good home.
References
2https://agrilifeextension.tamu.edu/library/gardening/essential-nutrients-for-plants/
3https://edis.ifas.ufl.edu/publication/AG462
4https://www.cdc.gov/chronicdisease/resources/infographic/chronic-diseases.htm
5https://www.nature.com/articles/nature16069
6https://2019.fertilizerreport.org/wp-content/uploads/2020/02/TFI-NutriFacts-Nitrogen_v2.pdf
8https://academic.oup.com/jxb/article/70/4/1119/5322156?login=false
9https://www.advancingecoag.com/plant-health-pyramid
10https://www.amazon.com/Nature-Properties-Soils-15th/dp/0133254488 (pg 644)
11https://www.ucsusa.org/resources/how-coal-works#sources
12https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-3040.2006.01614.x
13https://www.whoi.edu/cms/files/WaldronRobinsonnature2009_53167.pdf
14https://www.annualreviews.org/doi/10.1146/annurev-biochem-040320-101244
15https://link.springer.com/article/10.1007/s11104-021-05171-w
16https://content.ces.ncsu.edu/extension-gardener-handbook/1-soils-and-plant-nutrients
17https://nph.onlinelibrary.wiley.com/doi/pdfdirect/10.1111/j.1469-8137.2008.02751.x
18https://www.pnas.org/doi/full/10.1073/pnas.0712078105
20https://academic.oup.com/plphys/article/116/2/447/6085629?login=false
21https://link.springer.com/article/10.1007/s00203-022-03321-x
22https://www.ncbi.nlm.nih.gov/books/NBK209710/
23https://www.pnas.org/doi/abs/10.1073/pnas.2304663120
24https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4320215/
25https://annalsmicrobiology.biomedcentral.com/articles/10.1186/s13213-022-01701-8
26https://www.sciencedirect.com/science/article/abs/pii/B9780128183229000101
27https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10667278/
28https://www.sciencedirect.com/science/article/abs/pii/S0981942821003521
29https://springerplus.springeropen.com/articles/10.1186/s40064-016-3286-y
30https://www.sciencedirect.com/science/article/pii/S2452219817300459
31https://fungalbiolbiotech.biomedcentral.com/articles/10.1186/s40694-019-0086-5
32https://pubmed.ncbi.nlm.nih.gov/33103781/
33https://www.nature.com/articles/s41598-021-82768-2
34https://www.pnas.org/doi/10.1073/pnas.2017381118
35https://pubmed.ncbi.nlm.nih.gov/34657204/
36https://ohioline.osu.edu/factsheet/anr-37
37https://www.intechopen.com/chapters/85101
38https://link.springer.com/article/10.1007/s13199-021-00756-6
39https://nph.onlinelibrary.wiley.com/doi/pdf/10.1111/nph.13288
40https://pubmed.ncbi.nlm.nih.gov/18047587/
42https://ohioline.osu.edu/factsheet/sag-16
43https://www.sciencedirect.com/science/article/abs/pii/S0038071706000253
44https://www.sciencedirect.com/science/article/abs/pii/S0038071797002526
45https://amzn.to/3NcKLSC (pg. 498)
46https://onlinelibrary.wiley.com/doi/10.1002/ps.5527
47https://www.agric.wa.gov.au/measuring-and-assessing-soils/what-soil-organic-carbon
48https://agphd.com/resources/nutrient-removal-charts/corn-grain-and-stover-nutrient-removal-charts/
49https://www.sciencedirect.com/science/article/abs/pii/S0065211321001048
50https://uknowledge.uky.edu/cgi/viewcontent.cgi?article=1139&context=pss_facpub
51https://link.springer.com/chapter/10.1007/978-3-030-61010-4_1
52https://www.sciencedirect.com/science/article/abs/pii/S0038071720300390
53https://www.sciencedirect.com/science/article/abs/pii/S0167198711002030
54https://www.science.org/doi/10.1126/science.abe1148
55https://www.sciencedirect.com/science/article/abs/pii/S0006320718313636
56https://www.ecdysis.bio/_files/ugd/49b043_52b386bf17c644779508f99115975267.pdf
57https://conbio.onlinelibrary.wiley.com/doi/full/10.1111/cobi.12840
58https://biocontrol.entomology.cornell.edu/bio.php
59https://www.pnas.org/doi/10.1073/pnas.250483797
60https://link.springer.com/chapter/10.1007/978-3-540-74004-9_2
62https://www.sciencedirect.com/science/article/abs/pii/003807179190078X
63https://www.sciencedirect.com/science/article/abs/pii/S0167880923003675
64https://www.sciencedirect.com/science/article/pii/S0016706123000459
65https://ag.umass.edu/crops-dairy-livestock-equine/fact-sheets/plant-nutrients-from-manure
66https://extension.okstate.edu/fact-sheets/fertilizer-nutrients-in-animal-manure.html
67https://pubmed.ncbi.nlm.nih.gov/23128741/
68https://www.gov.mb.ca/agriculture/environment/nutrient-management/Pubs/properties-of-manure.pdf
69https://attra.ncat.org/publication/livestock-as-a-tool-improving-soil-health-boosting-crops-2/
70https://www.tandfonline.com/doi/abs/10.1080/21683565.2019.1591564
71https://www.sciencedirect.com/science/article/abs/pii/S0929139322002062
72https://www.nature.com/articles/s41598-022-06560-6
74https://carboncowboys.org/images/published_research/pdfs/apfelbaum-veg_infitration_carbon_2022.pdf
75https://www.jstor.org/stable/26453999
76https://www.sciencedirect.com/science/article/abs/pii/S0167880917300671
77https://www.nature.com/articles/nrmicro1750
78https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6584876/
79http://extension.cropsciences.illinois.edu/handbook/pdfs/chapter08.pdf
80https://www.tandfonline.com/doi/full/10.1080/00103624.2017.1407429
81https://besjournals.onlinelibrary.wiley.com/doi/10.1111/1365-2664.13833
82https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3946601/
83https://www.noaa.gov/what-is-harmful-algal-bloom
84 https://www.noaa.gov/news-release/below-average-gulf-of-mexico-dead-zone-measured
85https://pubmed.ncbi.nlm.nih.gov/24216420/
88https://pubmed.ncbi.nlm.nih.gov/35372745/
89https://www.fwi.co.uk/arable/crop-management/no-quick-fix-as-regen-ag-leads-to-lower-yields
90https://iopscience.iop.org/article/10.1088/1748-9326/aad401#fnref-erlaad401bib36
91https://rodaleinstitute.org/science/farming-systems-trial/
92https://acsess.onlinelibrary.wiley.com/doi/full/10.1002/ael2.20059
93https://ourworldindata.org/calorie-supply-sources
94https://peerj.com/articles/12848/
95https://www.mdpi.com/2073-4395/11/11/2278
96https://www.frontiersin.org/articles/10.3389/fsufs.2020.555426/full