4 Ecosystem Processes: Energy Cycle
What's the Matter?
How would you define the word “energy”? Pretty difficult, isn’t it? There are fewer words like energy that we use so often, but actually know so little about. There’s the U.S. Department of Energy, solar energy, wind energy, nuclear energy, clean energy, green energy and even energy drinks for when “I just don’t have the energy today…” The word energy is everywhere because it’s involved in every single activity a living organism or machine will ever do. Even processes that happen spontaneously without an input of energy, such as diffusion, involve energy. This is because we now know matter (anything with mass) and energy are just two sides of the same coin! A certain Albert Einstein described this relationship in his famous equation stating that Energy = Mass of an object times the speed of light squared, or E=mc2. What this means is that everything down to the level of the atom is just really, really concentrated energy. Case in point: “If you could turn every one of the atoms in a paper clip into pure energy—leaving no mass whatsoever—the paper clip would yield 18 kilotons of TNT. That’s roughly the size of the bomb that destroyed Hiroshima in 1945. On Earth, however, there is no practical way to convert a paper clip or any other object entirely to energy. It would require temperatures and pressures greater than those at the core of our sun.”1
Many smart people throughout the centuries have attempted to understand energy through experimentation and theories. One of the more interesting
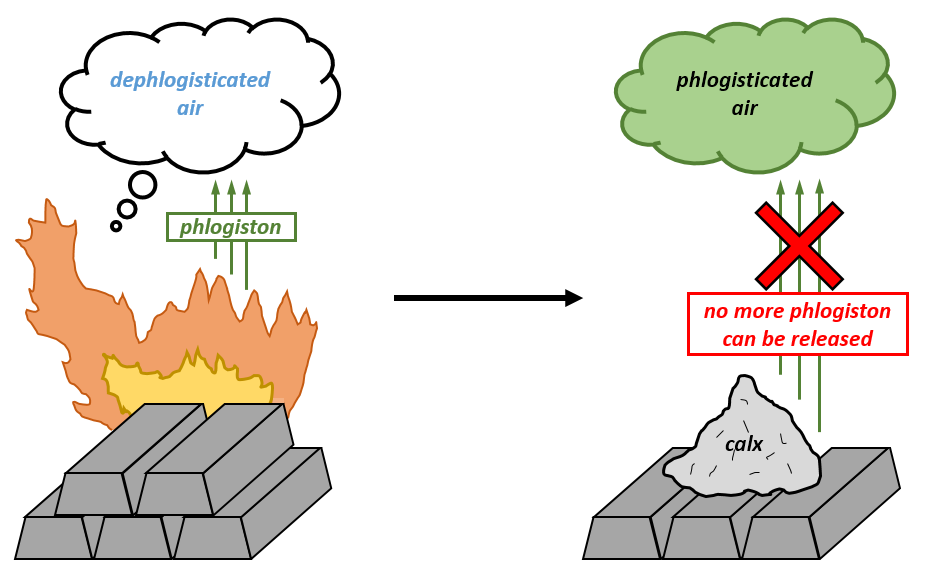
theories stated that flammable objects contain a substance called phlogiston and are “dephlogisticated” as it burns. True story. Despite being fun to say, the phlogiston theory was proven false and we now know that the light, heat and sound coming from a burning log is just escaped energy that was trapped inside the bonds of the wood the whole time. It requires some kind of oomph to free the trapped energy and get the log burning, as you probably know from the lack of logs spontaneously combusting all over the place. This oomph that has the ability to cause change is what scientists define as “energy”. Another way to say it is that energy enables something or someone to “do work”. Biological work includes the beating of our heart, dividing of our cells, contraction of our muscles and any other process living organisms can do. Abiotic work includes the heating up of brick by sunlight, a river moving a pebble downstream due to its flowing toward sea level, the falling of an apple from a tree onto Sir Isaac Newton’s head and many others. For the rest of this article, I will mainly discuss energy and work from a biological perspective.
That’s all fun and interesting, but why bring this up in a regenerative agriculture article? I bring it up because energy is just as important for land managers as it is for politicians, physicists and engineers. Farmers and ranchers are in charge of managing extremely complex biologically-driven systems that require constant inputs of energy to function properly. In fact, we may not think of it like this, but farming and ranching is essentially the process of capturing energy to grow stuff that we can sell. That’s really all there is to it at the most basic level. Therefore, it’s not a stretch to say that our businesses thrive, survive or nosedive depending on our management of the energy cycle. Hang with me through this science-heavy article because it will help you understand what energy is, how it flows in natural systems and how you can use it to your ecological and financial advantage.
For those who don’t want to get into the scientific weeds, skip down to the “Why Should I Care?” section to get the practical reasons why we should consider the energy cycle when making everyday farming and ranching decisions. You won’t hurt my feelings, but I believe you’d be skipping what I believe to be the scientific basis for why regenerative agriculture is not just the fad du jour. We don’t just imagine the benefits of regenerative agriculture. There are very real physical, chemical and biological principles of nature that regenerative agriculture seeks to align with. Conventional agricultural practices shortcut these principles largely through the use of cheap fossil fuels (a.k.a. ancient sunlight energy). Because we know fossil fuel supply is a limited resource, meaning prices will only go up from here, I would make the argument that modern conventional agriculture is the actual unsustainable fad. So, I encourage everyone to learn at least one or two facts prior to the “Why Should I Care?” section. I believe everyone is capable of understanding way more than they think they can, so why not give it a try? The more you know, the more you grow (literally and metaphorically).
Carbon is the Star of the Show
Sometimes we have the fortune of reading paradigm-shifting passages that truly change the way we view the world. The following quote from the textbook “Principles and Applications of Soil Microbiology” was one of those passages for me: “In discussing metabolic diversity [i.e. how living organisms obtain and use energy], a central concept is that the overall goal of all forms of metabolism is essentially the same —to obtain carbon and energy needed for the production of cellular constituents necessary for growth, survival, and reproduction.”2 From single-celled bacteria to nematodes to fungi to grasses to flowering plants to oak trees to elephants to our furry friends and finally to us, we all utilize strategies to obtain carbon and energy. This means metabolic differences between living organisms exist only in these strategies, not the overall goal! What a humbling experience it was for me to realize that I have the same energy goals as every other living creature on the planet. Maybe I’m just weird (in fact, I know I am), but I feel more connected to the natural world now that I understand the similarities between myself and all of life on the planet. We’re all just making the most of our environment and manipulating that environment as best we can to ensure growth, survival and reproduction. Even my view of disease-causing microbes, undesirable “weeds” and “pest” insects has changed dramatically. They’re simply opportunistic creatures looking to consume carbon and energy in a way that matches the strategies they’ve refined over time. The more that conditions match their preferences, the better they grow, survive and reproduce. This is why waging counterproductive war on individual species is the wrong strategy. Instead, designing and maintaining systems that feed a diversity of creatures that keep these “pathogens”, “weeds” and “pests” in balance with the rest of the system is a much more productive use of our time and energy.
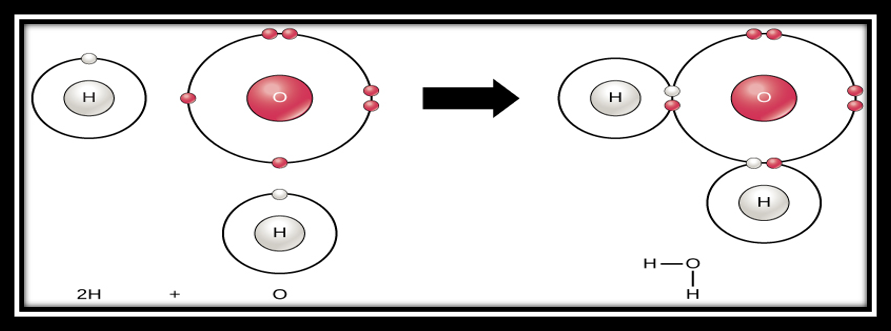
Ok, organisms need energy to do work. That makes sense. What, then, makes carbon so special and so sought after? Carbon is an atom like all other elements, and atoms are the basic building blocks of matter. Atoms are made up of negatively-charged electrons floating around a positively-charged nucleus. Atoms can share, lose or gain electrons to become more stable and/or create chains of atoms called molecules and compounds. I like to think of atoms as Legos constantly connecting and disconnecting from each other. As a general rule, atoms are most stable when they have two or eight electrons filling their outermost ring. Sharing an electron with another atom counts for the outer rings of both atoms. Take a look at the formation of water below to see that each hydrogen (H) atom ends up with two electrons on its outer ring and the oxygen (O) atom has eight electrons on its outer ring. The hydrogens and oxygen are extremely stable because they have full outer rings, which is why water likes to stay as water. If you’re confused, trace your finger around each of the circles and count the number of electrons on each circle regardless of whether they are white or red.
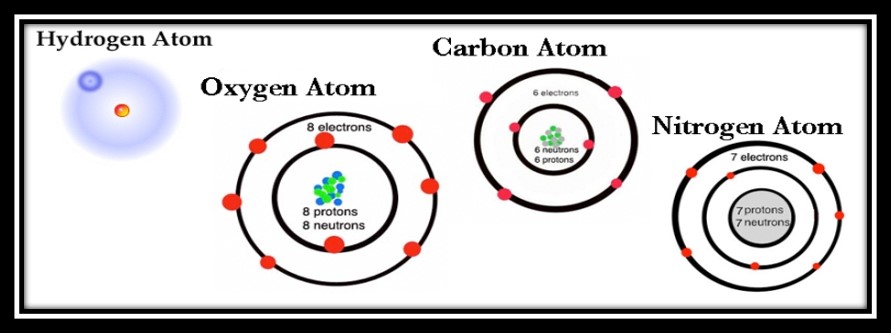
One factor that makes carbon so special is that it has four electrons on its outer ring. This means carbon has the opportunity to make four different connections with other atoms, as opposed to something like oxygen that can only make two before it’s satisfied. It’s a bit hard to imagine, but these atoms exist in 3-D, so the ability to make four connections with four different atoms means carbon can twist and turn and create all kinds of different shapes like straight lines, branched lines, spirals and rings. It’s also the smallest atom with four electrons on its outer ring, which is important because the bigger the atom, the bulkier it is. Bulky atoms aren’t able to form as many different shapes that organisms require. Check out the image below to see carbon’s four red electrons on its outer ring dying to pair up with other electrons to make a full eight.
Another reason why carbon is the star of the show is that carbon bonds really well with itself, but not so so tightly that it can’t break the bonds and form new bonds. It’s the perfect Goldlylocks situation. This means that carbon-to-carbon bonds are prime candidates to form the literal backbone of a near infinite amount of compounds on Earth, which is exactly what we see in nature. In fact, the proteins, fats, carbs, DNA and RNA in your body right now all contain carbon-based backbones. Now you can begin to understand why organisms on Earth are “carbon-based life forms” and why living organisms all have the same goal to obtain this crucial atom.
Gradients & Rules
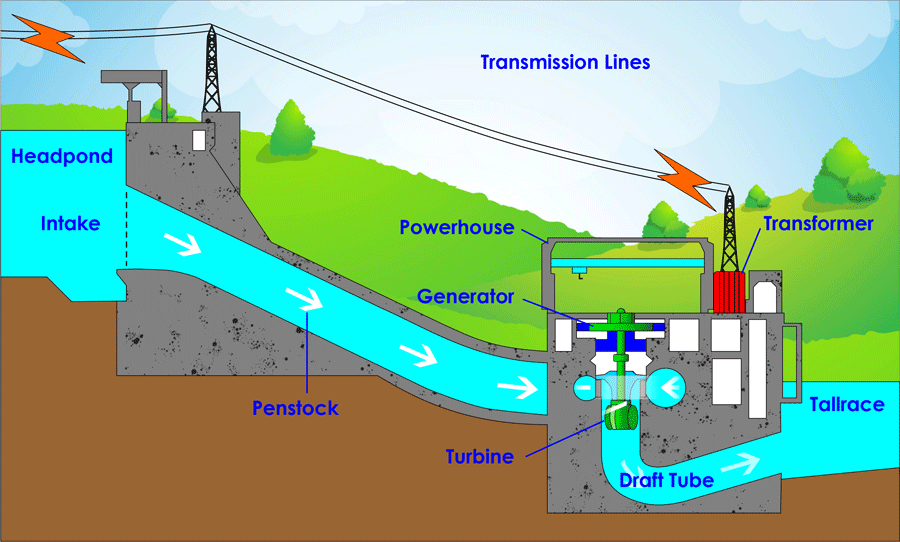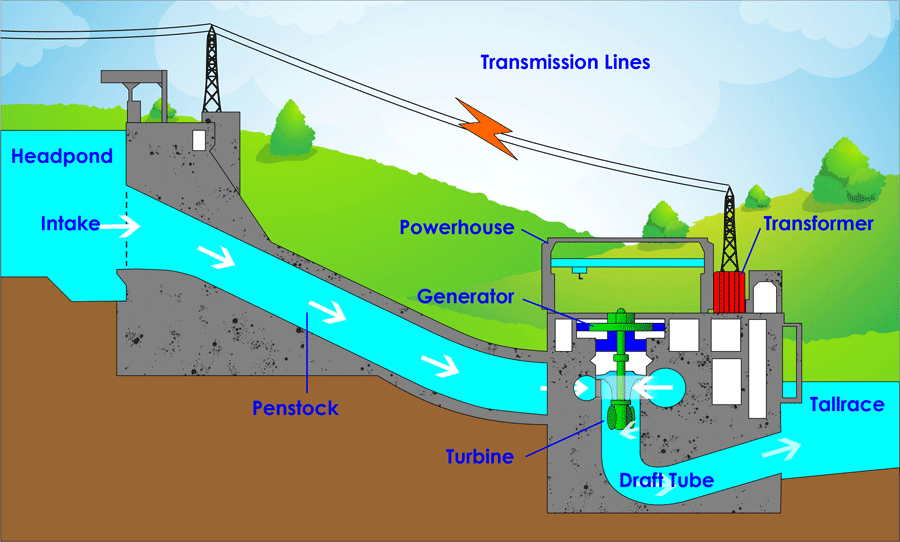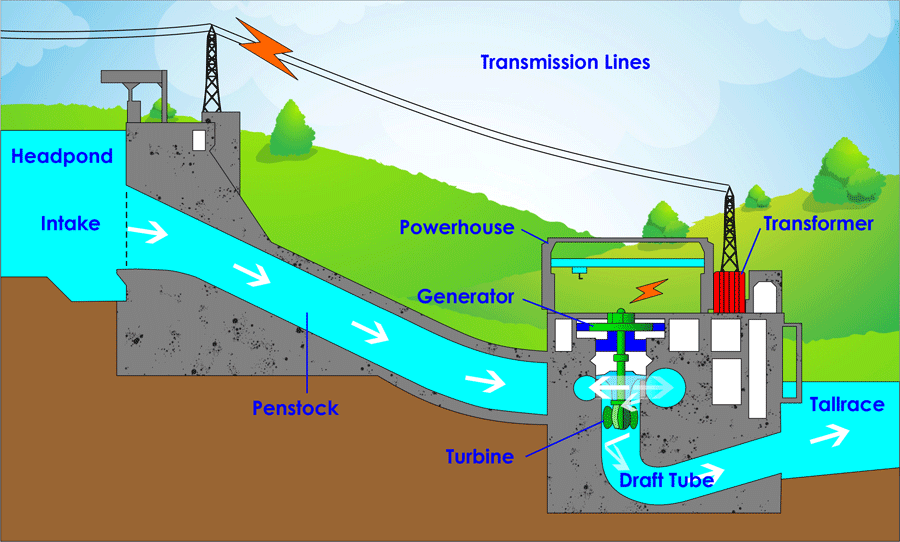
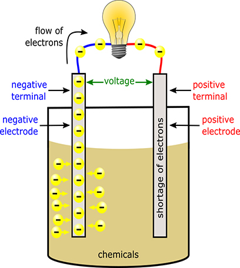
America may run on Dunkin, but the world runs on carbon. To understand why, we first need to realize that gradients are needed for energy to do work. A gradient simply means that there is more of something in one place and less of it in other places. After all, how fun is a roller coaster on a flat surface? Not fun because there is no gradient in height to allow gravity to do work on it! Hydropower also uses a height gradient to its advantage by allowing water to flow downhill and create usable energy by turning a turbine as it rushes past. Another gradient that is often taken advantage of is a concentration gradient. This means there are more physical objects in one area compared to other places. For example, electrons (the same electrons as the ones we discussed above) move from high concentration to lower concentration in a battery to produce an electric current that lights up a lightbulb or electronic device. Once these systems lose their gradient, whether by reaching a flat surface or an even distribution of objects, the ability for energy to do work reaches zero. This is a central concept of biology: living organisms have developed strategies to maintain gradients despite systems tending to even out over time and reach a stable equilibrium.
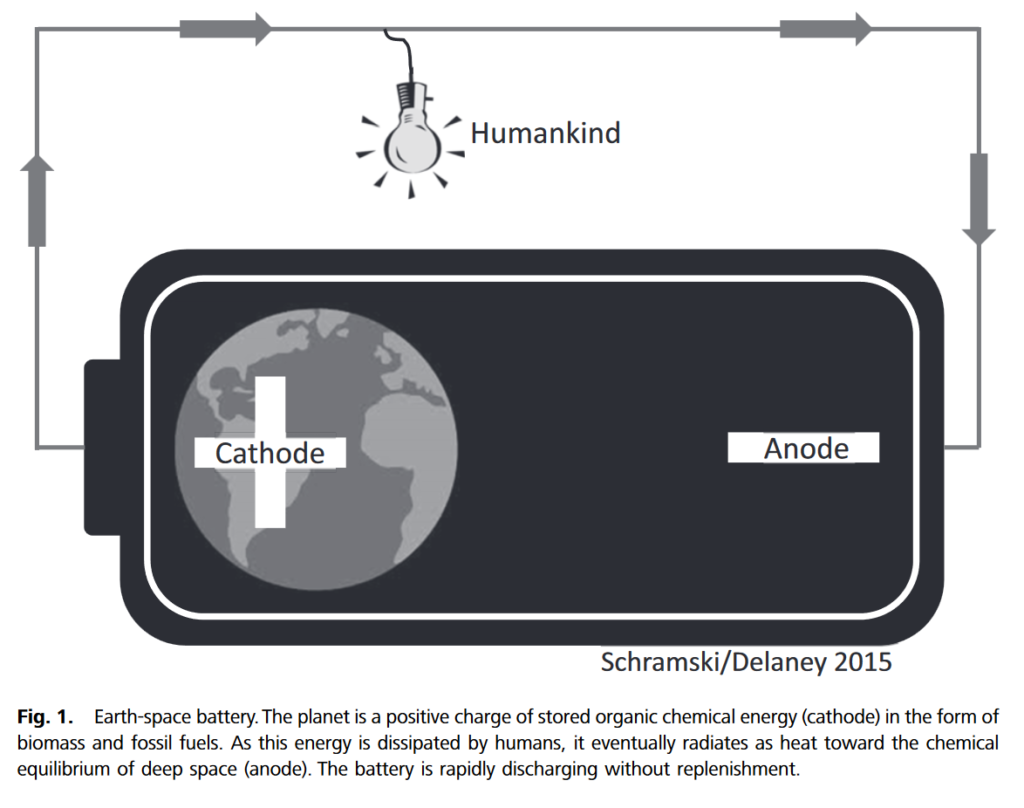
There is an excellent scientific paper from 2015 that describes the “Earth-Space Battery” we find ourselves living in. Basically, the Earth has enormous amounts of stored energy and outer space has none, forming a massive concentration gradient for work to be done. Their point is that we are depleting the charge of the battery quicker than we are currently recharging it, which is not sustainable. We will return to this idea later. For now, here is their definition of energy which includes the requirement for a gradient. They write, “Energy is how far a property (e.g., temperature, chemical, pressure, velocity) is from equilibrium. This distance, or gradient, can be harvested to perform work, in the process moving the property closer to equilibrium. Thus, whereas the capacity to perform work is often used as the simplest definition of energy, ultimately this capacity requires an out‐of‐equilibrium system, a gradient, which is available to be harvested.”3
To recap, energy can only make things happen when it exists in an out-of-equilibrium system. Flat roller coasters are no fun. Keep that in the front of your mind.
The next topic we must discuss before returning to carbon is how energy follows certain rules. These are called the Laws of Thermodynamics. I will discuss the 1st and 2nd laws as they are most applicable to farming and ranching. The 1st law states that energy in a closed system is neither created nor destroyed. Rather, it is conserved, meaning energy is just transformed from one version to another. As far as we know, our universe is a closed system, so energy is just transforming into one form or another, not increasing or decreasing. There are three types of energy in a system: kinetic (the energy of motion), potential (energy stored within a system as a result of placement or configuration), and internal (energy associated with electronic and intramolecular forces).4 In the case of hydropower, the height gradient gives water a quantity of “gravitational potential energy” because it is higher in elevation than the bottom of the system. That potential energy is transformed into kinetic energy when the water is placed on a downward slope, causing the water to move toward lower ground. The kinetic energy hits the turbine, transferring kinetic energy to the turbine, which causes it to spin. Through the use of generators and transformers, this kinetic energy is transformed into electric internal energy we can use to light up our houses. The amount of energy stayed the same throughout the whole process.
The 2nd law states that isolated systems, like our universe, are always moving towards disorder, or entropy as it’s known in physics. For example, entropy is low inside an ice cold diamond because the structure is very stable and the temperature is low, meaning the arrangement of molecules is largely set in stone (get it?). Compare that to a hot gas where the molecules have free reign to waft wherever they please and come into contact with whomever they please. The structure is disordered and has high entropy. Another way to describe entropy is to say that entropy is the amount of energy that is in a form that cannot do work. Here’s the key point: it turns out that every single transformation of energy on Earth loses usable energy, which most of the time is in the form of heat. This is why your car engine gets hot when it’s running and why you get hot during exercise. That heat is lost and is not in a usable form anymore.
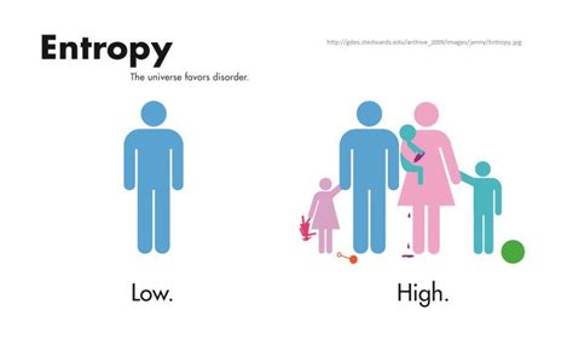
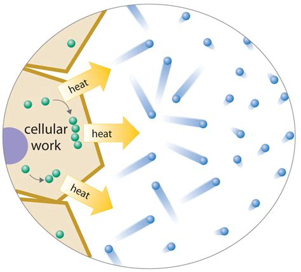
With ALL of that said, life inside of a closed system finds itself in a bit of pickle. Think about it. 1) New energy is not being created. 2) The amount of usable energy is decreasing with every moment of every day. 3) Energy can only make things happen when it exists in an out-of-equilibrium system. Uniform order, like in a frozen diamond, and uniform disorder, like in a cloud of heat, are in total equilibrium, which means energy cannot be used to make things happen in either case. So, if we can’t create new energy and the amount of usable energy for our purposes is constantly decreasing in an isolated system, how does life go on?
Here Comes the Sun!
Luckily for us, Earth is not a closed system. The star at the center of our solar system, which we call the sun, blesses half of the Earth with a continual stream of energy every second of every day. Remember when I mentioned that energy and matter are two sides of the same coin? Check out this video to hear Mike Rowe’s velvety voice describe the process of hydrogen atoms fusing inside the core of the sun, which turns matter into massless energy called photons. Photons band together to form electromagnetic waves (i.e. UV that gives us a sunburn, visible light which allows us to see colors and infrared which we feel as warmth) that leave the sun and reach the Earth after an eight minute, 19 second journey through space. This constant barrage of energy is what allows us to grow plants and animals that we can sell. Our job is to capture the sun’s incoming energy and convert it (1st Law of Thermodynamics) into energy that is usable for life. We must keep capturing the sun’s energy year after year because a portion of energy on our land is always transforming into unusable forms (2nd Law).
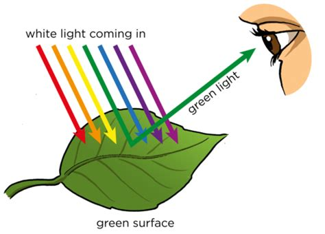
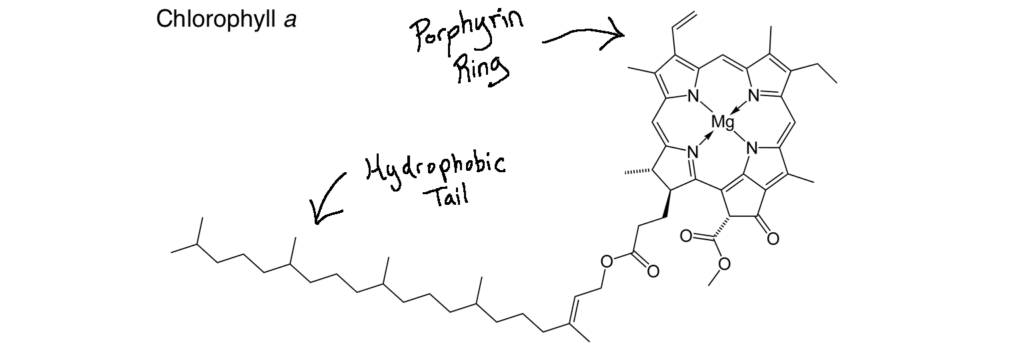
Enter photosynthesis. Sunlight energy is captured and transformed into usable energy by a few specialized organisms, such as cyanobacteria, algae, plankton and plants. While all of these amazing organisms deserve their moment in the sun, I will discuss photosynthesis from the perspective of plants as they are most relevant to farmers and ranchers. Plants, like all other photosynthetic organisms, contain special compartments in their cells called “chloroplasts“. Inside these chloroplasts are water, carbon dioxide vacuumed inside the leaf and molecules called “chlorophyll a & chlorophyll b” that absorb visible sunlight energy. Visible sunlight energy is part of the sun’s electromagnetic waves it emits and what allows our human eyes to perceive color. Sunlight actually contains all of the colors of the rainbow but appears white when they are all mixed together. We see color when this light is scattered away from each other, like blue in the sky during the day, or when molecules absorb certain colors and reflect others, which is exactly what chlorophyll does! Chlorophyll absorbs red and blue light well, while reflecting green light, which is why plants appear green to us.

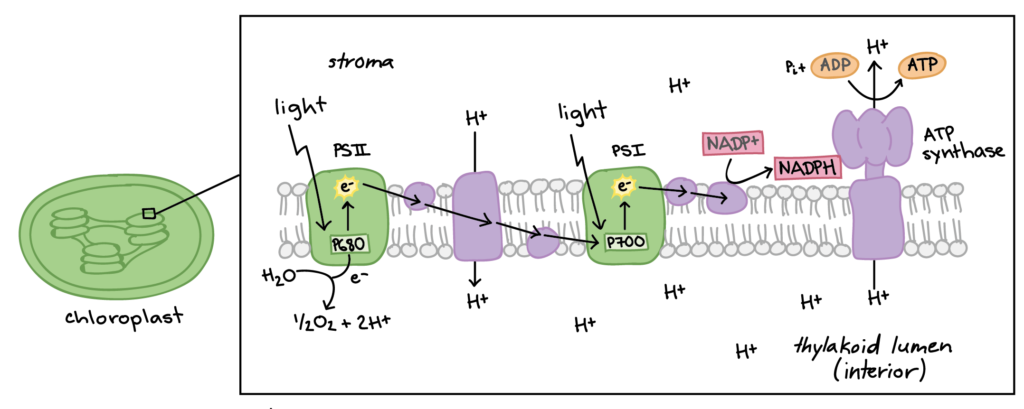

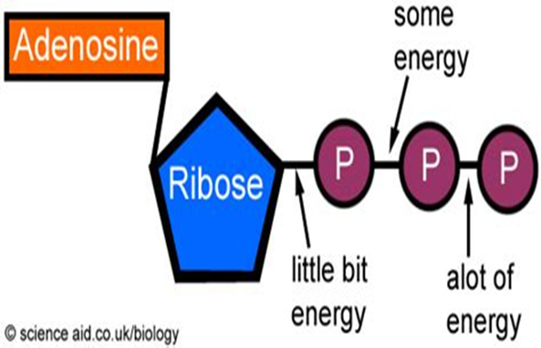
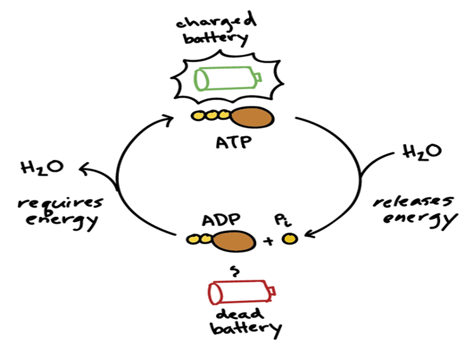
ATP, or adenosine triphosphate, is considered the energy currency for all of life. Whether we’re talking about microbes, plants, livestock or humans, being alive and staying alive requires a lot of energy. ATP are like mobile energy stations that can be transported to the site of an energy-intense process and release its energy to power said process. How does this work? The “tri” in triphosphate means that three negatively charged phosphate molecules have been crammed together. As you know from magnets, similar charges want to get away from each other, not get closer, so cramming them together can be thought of like the winding up of a spring. Once the ATP molecule arrives in the proper location, the final phosphate group shoots off and releases this pent-up energy, which is captured by the energy-intense process and allows it to proceed.
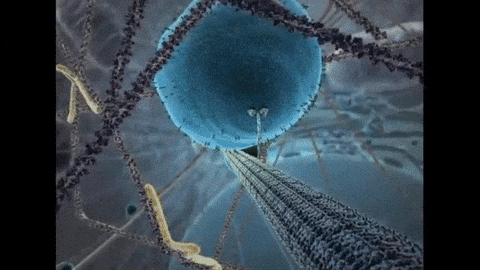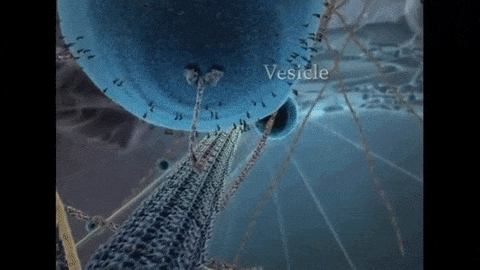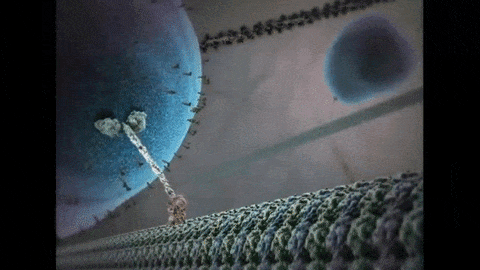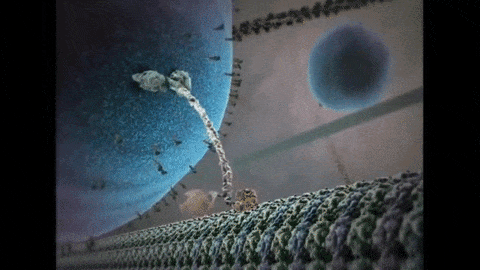
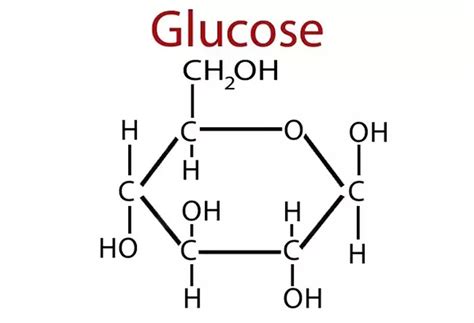
So far, I have described the first half of photosynthesis, which is called the “Light Dependent Reaction”. The second half of photosynthesis is called the “Light Independent Reaction” or the “Calvin Cycle”, which does not need light to proceed. As mentioned previously, plants vacuum carbon dioxide (CO2) gas from the air into their cells. Once inside the plant, the carbon and two oxygens are split from each other and the carbon is fused with a five-carbon chain to make a six-carbon chain. Energy from the first half of photosynthesis, via the electron captured by NADPH and the ATP that was produced, is donated to a chain reaction that results in the formation of our end product: glucose. Because biology is never simple, it actually takes six turns of the Calvin Cycle (input of 6 CO2, 18 ATP, and 12 NADPH molecules) to produce one molecule of sweet, sweet glucose. That’s a lot of time and energy to put into one molecule, so you know it must be worth it. After all, “Every action done in nature is done in the shortest way”, wrote Leonardo da Vinci.5
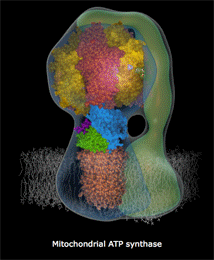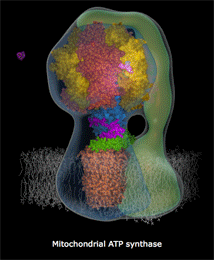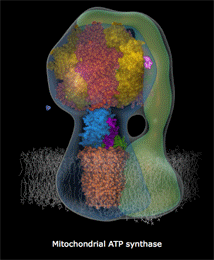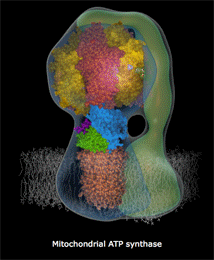
Now that the plant has forged its own food in the form of glucose, it can utilize glucose in many ways. Some individual glucose molecules are tasked with powering the production of ATP. This is accomplished by cracking glucose open and harvesting energy that originally came from the sun to turn that beautiful ATP synthase turbine we discussed earlier. This process is extremely efficient in the presence of oxygen (cellular respiration) and less so in the absence of oxygen (fermentation), which is one reason why plant growth slows in compacted or water-logged soils. Oxygen is quickly consumed and is unable to be replenished in both of those cases, which means root cells are starved of the oxygen they need for cellular respiration. Anaerobic microbes that are able to extract oxygen in creative ways, such as by splitting nitrate (NO3-) and sulfate (SO42-), run amok in this scenario, causing a gassing off of important nutrients, like nitrogen and sulfur. If you’ve ever dug in the soil and wondered why it smelled like rotten eggs, there’s your answer (H2S gas).
Plants can also fuse glucose molecules to one another to form long chains. These become the backbone of fat molecules, proteins and genetic material (DNA and RNA). One use for these long chains is to store the energy contained in glucose for when they might need it later, in what we call “starch“. Another handy use for these long chains of glucose is as building material. Cellulose, Earth’s most abundant chained structure, is the basic structural compound for plant cell walls. Hundreds to thousands of glucose molecules may be chained together in one cellulose molecule. Glucose can also be chained together to make hemicellulose, lignin (woody material) and pectin (which we take advantage of when we make jelly).
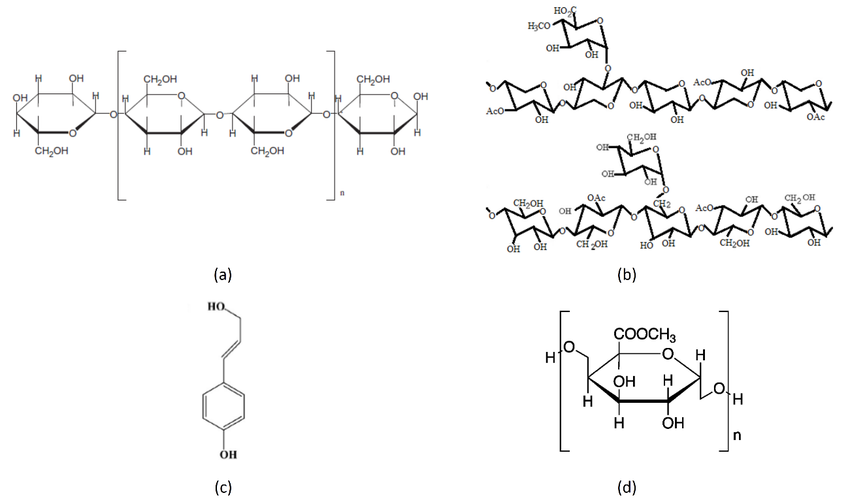
OK, let’s all take a deep breath because that was a lot of information. Here we go. In… out…. Great. The main point I’m trying to make is that life loses usable energy just by living. It’s a fact of life. We desperately need plants to capture as much of the sun’s FREE bombardment of energy on our land as they can. We then need them to place that energy inside molecules that living creatures can utilize. Otherwise, we slowly deplete the total amount of usable energy in a living system, such as soil, plants and animals. This is desertification in a nutshell. Deserts are low energy systems. They’re like those flat roller coasters we discussed earlier because equilibrium has been reached, usable biological energy is depleted and gradients cannot be formed, so no work can be done. The only work being done is by non-living factors, which is an extremely slow and inefficient process. Agriculturally speaking, practices that disrupt or remove growing plants, like tillage and fallowing, result in less sunlight energy captured for the system, which decreases the amount of usable energy, further decreasing the amount of living organisms that can capture sunlight energy in a vicious downward spiral. On the flip side, regenerative practices like cover cropping capture sunlight energy for more days of the year and make it usable for life, which leads to more life that will capture even more energy, starting a beneficial upward spiral of compounding and cascading events. To me, this energy cycle is the foundation of regenerative agriculture and is precisely what we are seeking to regenerate.
The Carbon Cycle
Now that we understand plants have the ability to turn carbon dioxide and sunlight into food, it’s time to think about how this carbon moves throughout the landscape. As it turns out, the entire carbon cycle is made up of two parts: the “Fast Carbon Cycle” and the “Slow Carbon Cycle”. The Fast Carbon Cycle refers to carbon that is quickly transferred between living organisms and the atmosphere. Let’s enter the cycle with a newly minted 6-carbon glucose molecule in the leaf of a plant. This glucose molecule will one day be ingested as food and cracked open for its energy either by 1) the plant, 2) a microbe 3) an animal, or 4) a fire. It may surprise you, but the process is the same in all four cases. Each utilizes oxygen in what is known as a “combustion” reaction, giving literal meaning to the term “burning calories”! Combustion of glucose releases energy for the organism to create ATP, the energy currency of life, as well as carbon dioxide and water, which is what we (and plants and microbes and fire) exhale. Of course, some carbon that we eat is retained and used as building blocks for cells. However, the same fate eventually befalls this carbon as something or someone will combust it and release it as carbon dioxide sooner or later. For example, as plant residue is combusted by soil microbes as food, a good portion of the carbon is exhaled as carbon dioxide gas, which rises toward the sky.

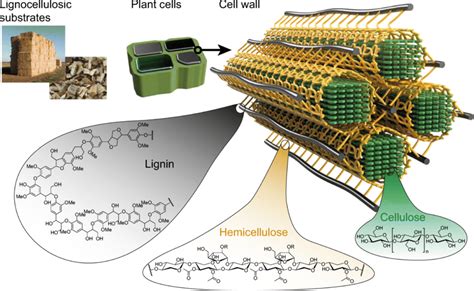
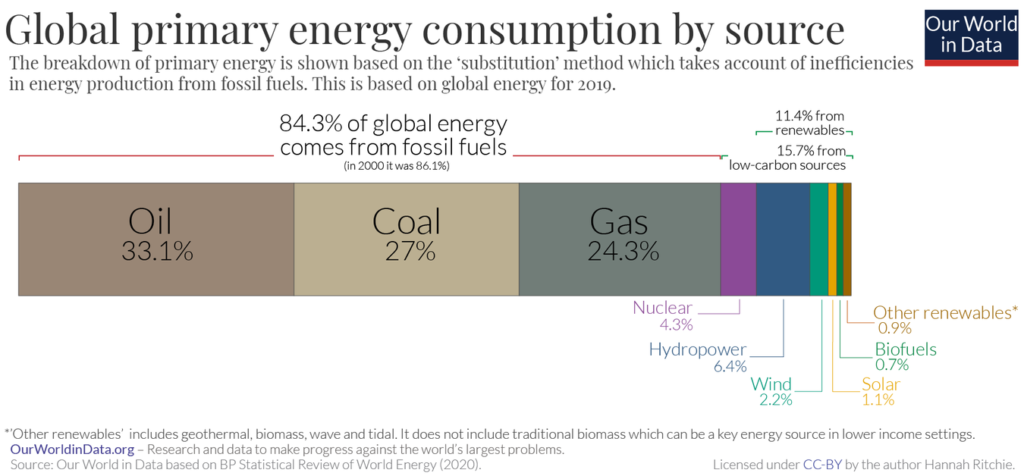
If you’re still on the fence about all of this carbon mumbo jumbo, remember that the world runs on carbon. We need the Fast and Slow Carbon Cycles functioning properly to continually provide us with usable energy and friendly living conditions. Human advancement depends on it. Abe Collins, board member of the Soil Carbon Association, said it well: “Every major advance in human economic and social life has been tapping a new carbon source. Think of farming, oxidizing organic matter to release nutrients to grow crops, think of forestry –for timber and charcoal, think of coal, and then oil.”7 The stats back this up. In 2019, over 84% of global energy consumption came from oil, coal and natural gas. Carbon is by far and away the most common element in these fossil fuels, as you can see in the images below.
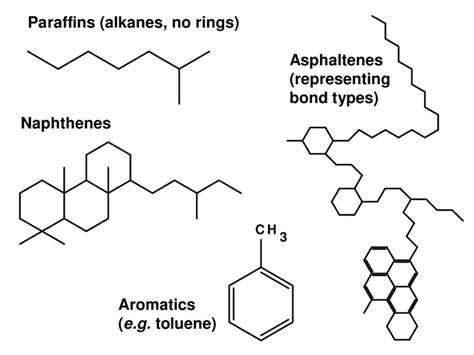
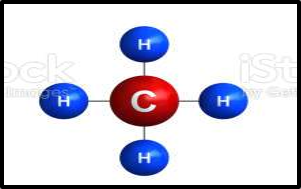
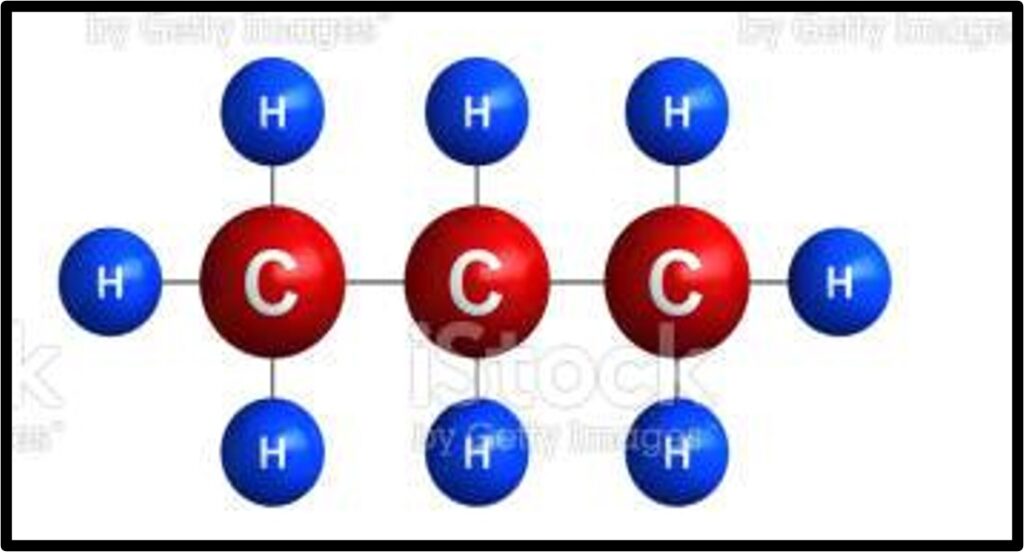
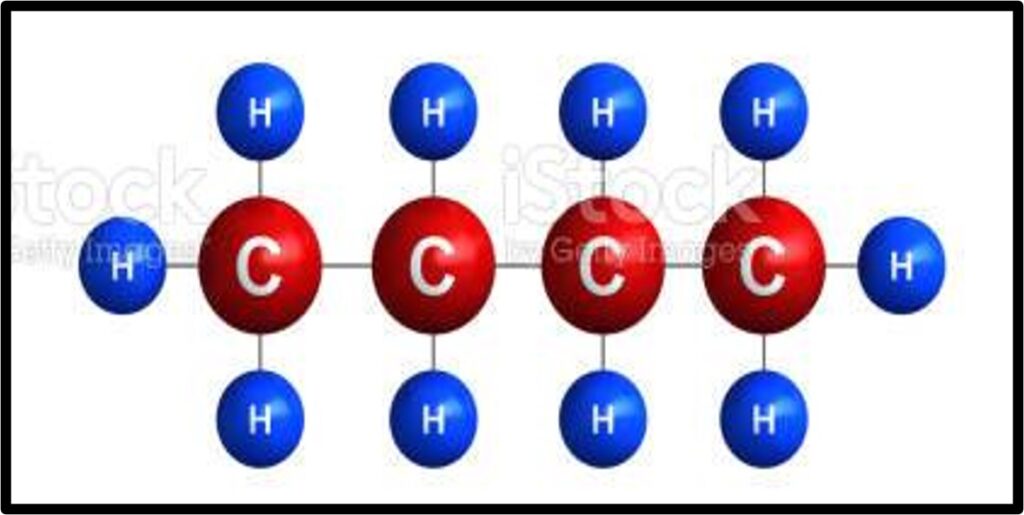
Extracting and refining this ancient photosynthetic energy into a form our modern technology can combust is time-intensive and expensive, not to mention it takes millions of years to make more of it. Humanity will need to transition away from ancient energy as its main energy source at some point, whether that’s in 10 years or 500 years. Which energy source we transition to will likely vary by sector. Through the miracle of photosynthesis, our food system has the unique opportunity to be an industry that derives its energy from the cleanest, most sustainable power source there is: the sun. Agriculture has the potential to be a shining city upon the hill if we prioritize working with nature and not against her. The opportunity is beckoning.
Why Should I Care?
I understand if all of this seems boring or like it’s too deep in the weeds to be practical. Most farmers and ranchers like to be outside working with the land and their animals, not so much with their noses buried in books at the library. But the fact is that agriculture is one of the few remaining industries that works directly with natural systems, and natural systems run on energy. Our job is to capture and transform an enormous amount of energy to grow a plant- or animal-based product that can then be sold. In wealthy nations like in the United States, we’re blessed with incredible infrastructure that can deliver fossil fuel-derived products, such as diesel, fertilizers and pesticides, at relatively affordable prices, although that is changing. In other words, ancient sunlight energy is delivered right to our doorstep. However, fossils fuel energy is not a long-term solution for food production systems. Conventional agricultural practices are also unsustainable as they lead to enormous losses of carbon and energy from the soil profile, which, in turn, increases the demand for imported ancient sunlight energy. One study estimates that some cultivated plots around the world have lost one-half to two-thirds of the original soil organic carbon pool.8 Another found that 35 ± 11% of the carbon-rich topsoil has been completely removed from cultivated area of the U.S. Corn Belt.9
Luckily for us, we have a glowing fireball in the sky that bombards the land with FREE packets of energy for our entire lives. Even luckier is that it won’t run out of fuel for billions of years. Therefore, there is a huge opportunity for land managers to capture more of this energy and place it inside the cells of living beings that can do work for us. The more energy we can capture and put to good use, the less energy we need to purchase in the form of diesel, fertilizer and pesticides. It just makes good business sense. If someone offered you free energy, wouldn’t you at least investigate it to see if it works? Wouldn’t you ask others that have chosen to use this free energy source whether or not it works? There are many farmers and ranchers around the country that have put this knowledge into practice and are reaping the financial rewards.10 Search for YouTube videos of Gabe Brown, Allen Willliams, Rick Clark, Greg Judy, Joel Salatin and many others to find the common principles they teach that made them profitable. In my opinion, the common thread connecting successful regenerative farmer or ranchers is that they have figured out how to get their operation to run more on FREE sunlight energy and less on expensive sources of old sunlight energy. Let’s break down a couple of the reasons why we import ancient energy onto the farm and how we can substitute modern solar energy do those same jobs.
First, plants require nutrients to grow. To get these nutrients inside of plants and eventually into livestock, land managers must decide how much energy will come from 1) mining, refining, transporting and applying fertilizer to the land or 2) natural nutrient cycling done by living organisms powered by modern sunlight. The latter is how natural systems operate because nobody fertilizes the great rainforests, savannas and grasslands of the world, and yet they are the most productive landscapes on the planet. How can this be? Natural landscapes support healthy plants that are able to photosynthesize efficiently, which feeds soil biology with carbon and energy in exchange for nutrients they scavenge. Realize that 94% of a plant’s weight is made up of carbon, hydrogen and oxygen, while nitrogen makes up of around 3-4% of healthy plants. Conveniently, all four of these elements are found for FREE in air and water. For example, nitrogen makes up 78% of air in the form of two nitrogen atoms triple-bonded to each other. This means there are 32,000 tons of FREE nitrogen bathing an acre of plants at all times! The trouble is that triple-bonded nitrogen is extremely stable and requires a lot of energy to transform it into a form that organisms can use. Incredibly, microbes in the soil called diazotrophs, which includes rhizobia that team up with legumes, receive carbon and energy by splitting these nitrogen atoms apart. These microbes produce the right compounds to split up the nitrogen atoms, splice them to other atoms and provide them to the plant in exchange for photosynthetic carbon and energy. For non-legumes, there are many different kinds of free-living diazotrophic microbes that will gladly share their nitrogen bounty in exchange for carbon and energy. Therefore, the best way to fertilize your land with nitrogen is to aerate the soil so these solar powered microbial nitrogen fixers can come into contact with the literal tons of free-floating nitrogen in the air.
To recap, up to 97% of a plant’s weight comes from FREE air and water, which can be manufactured into plant material by FREE sunlight energy. It makes sense that life is designed to take advantage of the most abundant elements they’re bathing in! The remaining nutrients that make up 3% of a plant’s weight, like phosphorus, potassium and sulfur, are initially released out of rocks and minerals. Once the nutrients are released by plants, microbes or weathering, they are taken up and recycled by growth and decay cycles. Read about the Nutrient Cycle to learn the full story, which includes discussions on organic matter, micro-manure, nutrient mining and even carnivorous plants.
Second, plants need to be in an overall state of health to avoid unwanted consumption. Microbes and insects are always on the look-out for carbon and energy sources to consume. When they spot a weak individual, they take their chance and cause what we call “infections”, “outbreaks” and “disease”. They call it a digestible lunch. The need for energy-intensive pesticides is an indication of plants and animals with subpar health and suppressed immune systems. Physician Lionel Picton details this in his book Thoughts on Feeding. He writes, “Fresh food, grown [on fertile soil] confers upon the animals and men that consume it the powers of resistance to germs whose function it is to prey upon and even eliminate men and animals who are devoid of those powers.”11 Microbes and insects are just doing their job, which is to spot food they can digest and leave the rest alone. To ensure that plants and animals aren’t picked apart by opportunistic microbes and insects, land managers must decide how much energy will come from 1) mining, refining, transporting and applying pesticides to the land or 2) symbiotic relationships between living organisms powered by modern sunlight. Once again, the latter is how natural systems operate because nobody applies chemical pesticides to the great rainforests, savannas and grasslands of the world, and yet they are the most resilient and healthy landscapes on the planet.
The secret to mimicking resilient landscapes on our farms and ranches begins by providing enough carbon and energy to the trillions of soil microbes interacting with plants. A rich diversity of microbes provides the plant with protection from pathogens in many different ways, including chemical warfare (release of antibiotic and antifungal compounds), competition for resources, predation and parasitism.12 In addition, relationships with common soil microbes like Pseudomonas, Bacillus, Trichoderma, and mycorrhiza species stimulate a plant’s immune system without directly activating defenses that cost the plant a lot of energy that could have been used for growth or nutrient acquisition. These disease-supressive benefits are one of many reasons why plants exude up to 40% of their photosynthetically fixed carbon and energy out into the soil. They are creating a favorable environment around their roots by making it one of the most energy-rich habitats on Earth.”13 This begins a cycle of health that leads to healthy, nutrient-dense plants that confer health to the livestock that consume them. Human health ultimately benefits by consuming these nutrient-dense plants and animals. None of that is possible without consistent streams of carbon and energy into the soil by growing plants.
The last important energy-intensive job that I will touch on is water holding capacity and water uptake. Plants need water and lots of it. So do microbes, worms, sheep and all other living organisms. To ensure that everyone on the farm has sufficient access to water, land managers must decide how much energy will come from 1) drilling, pumping and applying irrigation to the land or 2) soil structure building by living organisms powered by modern sunlight. Unsurprisingly, water infiltration and availability greatly increase when the soil is provided with sufficient inputs of carbon and energy14. Click here to get the full scoop on the Water Cycle.
As you can see, increasing the size and charge of a farm or ranch’s battery is done through the addition of photosynthetic carbon and energy. A bigger battery means more jobs done for you at a much lower cost. This is regenerative agriculture in a nutshell. Research on this subject is in its infancy, but let’s put some numbers to it. Plants that produce 1 kg of glucose are capturing and storing 16 megajoules of sunlight energy in a biochemical form. Bare soil has no way of capturing these joules of energy, so sunlight that strikes the soil surface is simply lost as heat that is radiated back into the atmosphere.7 Think about this wasted energy the next time you see a field with no plant cover. I also encourage our readers in heavily cropped areas to think about how many days of the year their fields are covered with green, growing plants that are pumping carbon and energy into the soil. In a typical corn/soybean rotation, the answer is less than 25% of the time. In addition, think about how much of a field’s soil surface is covered with green, growing plants on June 21st, the day with the most sunlight in the Northern Hempisphere. Observe natural areas around you on June 21st and you will likely see plant growth in woods and prairies are in sync with this max input of energy, while cropping fields are not. It’s important to note that pastures vary greatly in their ability to pump carbon and energy downward, so it’s not just cropping systems that need to be more conscious of their use of sunlight. Research comparing five adaptive multi-paddock grazing (AMP) systems with five conventionally grazed systems found that AMP grazing led to significantly more standing crop biomass, which resulted in a whopping 46% higher pasture photosynthetic capacity.15 Every operation has room for improvement.
Another energy benefit that comes from regenerative practices is increased populations of soil fungi16, which are more effective at storing carbon and keeping it on the farm or ranch compared to bacteria.7 This means fields and pastures that have a severe lack of soil fungi, as most of our conventionally managed fields and pastures do, lose a higher percentage of their organic matter to carbon dioxide as it gases off. Soil fungi are also crucial in building macroaggregation17, which protects organic matter from quick degradation.18 Organic matter is chock full of usable energy because it is composed of dead and decaying life composed of carbon-rich compounds. This means building up organic matter levels should be a top priority for producers looking to increase the size and charge of their operation’s battery.
I’ll end with a thought-provoking quote that you can read in the image below. The statement is from the father of American wildlife ecology, Aldo Leopold, author of the famous 20th century book A Sand County Almanac. Sit with his words for a few moments to see if you agree or disagree. Is land a fountain of energy or is it just lifeless dirt that holds nutrients? Spending a minute or two pondering Aldo’s quote just might save you thousands, hundreds of thousands or even millions of dollars over your lifetime if it leads to a more energy-efficient operation. Talk about a good ROI.

Summary
The next time you’re thinking about crop yield or pounds produced, I encourage you to consider energy yield and energy production as well. How much sunlight energy are you capturing and trapping in the ecological system you manage? How much life are you producing with the sunlight that hits your land every year? Are you wasting a lot of it? Why do you have to import so much energy onto your operation to keep the system moving? Why don’t Earth’s most productive lands, the forests, savannas and prairies, have to import old sunlight energy? Can your farming practices mimic the energy efficiency of these systems? Over the past century or so, farmers and ranchers have outsourced their thinking of such topics to agronomists, salespeople and chemical companies, who often don’t have their best interest in mind. Some do, many don’t. Understanding the energy cycle gives producers the tools to figure out who is for them and who is just trying to make a buck off of them.
The call to action goes for all of us, not just those of us in agriculture. Have a lawn? Well, the University of Massachusetts found that the typical lawn-service company in that state applies five to seven pounds of pesticides per acre of lawn a year, which is more than twice the amount applied to sweet corn.19 Golf courses can be worse.20 At the very least, we should all become conscious consumers of food when we are able. Look for regenerative food labels, like the Regenified label, to ensure that your food was grown from modern solar energy in an energy-efficient manner. We all have a role to play in maximizing the energy efficiency of modern systems, not just the producers of our food, fuel and fiber.
P.S.
I must admit that I have a confession: I intended to make this article very science-heavy on purpose, which I know might not be everyone’s cup of tea. As someone who loves to learn science and finds that the natural world is fascinating beyond belief, I wanted to make a point. In this small article, I discussed how subatomic physics, chemistry, biology, ecology, engineering, anatomy, pathology, economics, and many other fields all directly apply to agriculture. Not many industries can boast that kind of variety. Our best and brightest students are very often pushed toward white collar occupations that are deemed “highly intelligent”, like medicine, physics, chemistry, law, computer science, while simultaneously pushed away from blue collar “dumb” jobs, like farming, plumbing, construction, mechanic work, etc. I hope that I’ve shown that the energy cycle is intelligent, scientific and relevant to the farmer or rancher, which proves that agriculture is not just thoughtless drudgery for the stupid rural people of our societies. With the average age of American farmer at 57.5 years old21, we need to be thinking about who is going to replace this aging workforce. Young people want work that’s fulfilling, intellectually stimulating, innovative, helpful to the environment, good paying and provides the freedom to be your own boss, just to name a few. We are doing ourselves a major disservice by failing to portray agriculture, specifically regenerative agriculture, as an industry that checks all of those boxes more than any other industry. Yes, farming and ranching is hard work and can be isolating at times. It’s not for everyone. However, I believe that a large number of young people will want to move back to the farm or ranch to fill open jobs when we accurately portray agriculture as the deep-thinking, scientific, purposeful endeavor that it truly is.
References
1https://www.pbs.org/wgbh/nova/einstein/lrk-hand-emc2expl.html
2http://dx.doi.org/10.1016/B978-0-12-820202-9.00003-4 (Chapter 3)
3https://www.pnas.org/doi/full/10.1073/pnas.1508353112
5https://archive.org/details/noteboo00leon/page/n7/mode/2up
6https://www.earthobservatory.nasa.gov/features/CarbonCycle
7https://amzn.to/3oZLYUw (pg. 26)
8https://www.sciencedirect.com/science/article/abs/pii/S0016706104000266
9https://www.pnas.org/doi/full/10.1073/pnas.1922375118
11https://www.amazon.co.uk/Thoughts-Feeding-Lionel-Picton/dp/1904665101
12https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7953945/
15https://peerj.com/articles/13750/
16https://www.tandfonline.com/doi/abs/10.1080/21683565.2019.1591564
17https://www.nature.com/articles/s41396-019-0369-0
18https://www.sciencedirect.com/science/article/abs/pii/S0065211316301134
20https://www.theguardian.com/us-news/2022/aug/06/pesticides-golf-courses-health-problems
21https://fortune.com/2023/10/12/how-old-american-farmer-average-age-boomer-gen-x/